-
PDF
- Split View
-
Views
-
Cite
Cite
Viviane Gonçalves Leite, Finn Kjellberg, Rodrigo Augusto Santinelo Pereira, Simone Pádua Teixeira, What makes a fig: insights from a comparative analysis of inflorescence morphogenesis in Moraceae, Annals of Botany, Volume 127, Issue 5, 9 April 2021, Pages 621–631, https://doi.org/10.1093/aob/mcaa202
Close - Share Icon Share
Abstract
Moraceae, the family of mulberry and fig trees, displays small homogeneous flowers but extremely diverse inflorescences ranging from simple and branched to complex and condensed. Inflorescences also vary in flower organization in the receptacle, in the degree of flower condensation and in receptacle shape. Thus, the objective of the present study was to compare the inflorescence morphogenesis of Moraceae species, to investigate whether clades with a similar pollination mode share the same patterns of inflorescence development and the developmental stages at which we observe the key changes resulting in the diversified inflorescence architecture that culminates in the Ficus syconium.
Inflorescences at different developmental stages were sampled from Brosimum gaudichaudii, Castilla elastica, Clarisia ilicifolia, Ficus pertusa, Maclura tinctoria and Morus nigra and processed for surface and anatomical analyses.
The inflorescence morphogenesis of the studied species is highly variable. The shape of the inflorescence meristem (bulging, hemispheric or elongated), the initiation order and arrangement of flowers along the receptacle and the occurrence of bracts vary between related species. This diversity originates early during inflorescence development. Brosimum gaudichaudii, C. elastica and F. pertusa have flowers enclosed or immersed within the receptacle, although inflorescences begin their development as flat and open structures, as occurs in the other three study species.
Comparison of the inflorescence morphogenesis in Moraceae species allows us to infer that evolutionary ontogenetic changes driven by pollinators culminate in the enclosure of flowers inside the receptacle, as occurs in the Ficus syconium.
INTRODUCTION
The diversity of floral morphology and inflorescence architecture within angiosperms illustrates the extreme evolutionary plasticity of their reproductive structures (Harder and Prusinkiewicz, 2013). Although floral morphological diversity has been described for more than two centuries (Baker, 1983) and characterized since Darwin (1862, 1877), inflorescence morphology has received much less attention (Wyatt, 1982; Harder et al., 2004; Prusinkiewicz et al., 2007; Harder and Prusinkiewicz, 2013). This is surprising since, taken together, flowers and inflorescences often constitute functional units for pollination, fruit development, attraction of frugivores and seed dispersal (Harder and Prusinkiewicz, 2013). Inflorescence shape is constrained by developmental, genetic and environmental factors (Singer et al., 1999; Tucker and Grimes, 1999) that will condition its response to the selective pressures exerted by pollinators (Ishii et al., 2008). Inflorescences are classified according to several criteria, such as ontogeny, shape and size of the axis (= receptacle), and occurrence and size of a peduncle (Rickett, 1944; Weberling, 1965, 1988, 1989; Bell, 1991; Greyson, 1994; Weberling and Troll, 1998; Singer et al., 1999; Tucker and Grimes, 1999; Prenner et al., 2009; Endress, 2010; Claßen-Bockhoff and Bull-Hereñu, 2013; Kirchoff and Claßen-Bockhoff, 2013).
Moraceae, the family of mulberry and fig trees, displays small homogeneous flowers but extremely diverse inflorescences ranging from simple and branched to complex and condensed (Berg, 2001; Datwyler and Weiblen, 2004; Ribeiro, 2007). Inflorescences also vary in terms of flower organization in the axis/receptacle, in the form and degree of condensation/union of the flowers, and in the shape of the receptacle, which is reflected in the diversity of inflorescence types and shapes (racemes, spikes, cyme, syconium, head, urceolate receptacles; Clement and Weiblen, 2009) and even in the types of infructescence (Berg, 2001). Such extreme variation is accompanied by a wide variation in breeding systems within the family (monoecious, dioecious, andromonoecious, gynodioecious), and inflorescences may show pistillate and/or staminate flowers, even in monoecious species (Berg, 2001; Berg et al., 2006; Ribeiro, 2007; Clement and Weiblen, 2009; Zerega and Gardner, 2019). Moraceae encompasses ~1100 species, 39 genera (Berg, 2005; Berg et al., 2006; Clement and Weiblen, 2009) and seven tribes (Artocarpeae, Castilleae, Ficeae, Dorstenieae, Maclureae, Moreae and Parartocarpeae; Zerega and Gardner, 2019). Thus, its representatives are excellent models for studies of the functional diversity of reproductive structures. Investigating their inflorescence ontogeny may provide insights into selective pressures that shaped the floral moracean structures.
Inflorescence architecture in Moraceae may vary within and among taxonomic levels (i.e. species, genera and tribes) (Berg, 2001; Berg et al., 2006; Ribeiro, 2007; Clement and Weiblen, 2009; Zerega and Gardner, 2019). In Maclureae, for example, the staminate inflorescences can be spicate, racemose or globose, whereas the pistillate inflorescence is globose (Clement and Weiblen, 2009). On the other hand, the syconium, an urn-shaped inflorescence that encloses diclinous (= unisexual) flowers densely arranged on the inner surface of the receptacle, with an apical opening called the ostiole, is exclusive to Ficus (Clement and Weiblen, 2009). Similarly, discoid inflorescences, containing diclinous flowers immersed in the receptacle, are restricted to Dorstenieae (Berg, 2001; Clement and Weiblen, 2009). Despite the diversity of inflorescence architecture and richness in species (Berg, 2005), few studies on the inflorescence development of this family are available (Baillon, 1861; Golenkin, 1894; Bernbeck, 1932; Moncur, 1985).
From a series of morphological traits and published pollination observations, we may infer that Moreae is generally wind-pollinated, Artocarpeae is pollinated by gall midges that breed in staminate inflorescences, Maclureae is wind-pollinated, Dorstenieae can be pollinated by flies that breed in the inflorescences or wind-pollinated, Castilleae is pollinated by thrips that breed in staminate inflorescences, and Ficeae is pollinated by agaonid wasps that breed in the urn-shaped inflorescences (Berg and Hijman, 1999; Sakai et al., 2000; Sakai, 2001; Kjellberg et al., 2005; Taylor et al., 2006; Araújo et al., 2017). Hence, available data suggest that Moraceae are either wind-pollinated or are involved in a series of biologically varied brood-site pollination mutualisms. We propose that different types of brood-site pollination mutualisms select for divergent inflorescence structures. Comparing the development of such diversified inflorescences may provide some insights into the morphogenic control of floral traits.
Thus, the objective of the present study was to compare the inflorescence morphogenesis of Moraceae species in order to investigate the role of pollination mode on the inflorescence development that culminates in the Ficus syconium. For this purpose, we compared the inflorescence development of wind- and insect-pollinated species, seeking to key out developmental changes associated with the inflorescence architecture.
MATERIALS AND METHODS
Six species representing six of the seven recognized tribes were sampled: Morus nigra, tribe Moreae (elongated raceme, oval spike); Clarisia ilicifolia, tribe Artocarpeae (elongated spike, globose head); Maclura tinctoria, tribe Maclureae (elongated spike, globose head); Brosimum gaudichaudii, tribe Dorstenieae (globose head); Castilla elastica, tribe Castilleae (bivalvate, urceolate, discoid heads); and Ficus pertusa, tribe Ficeae (urn-shaped syconium) (Fig. 1, Table 1). Representatives of C. ilicifolia, M. tinctoria and B. gaudichaudii are naturally found in Brazilian vegetational formations (Berg, 2001). Morus nigra, F. pertusa and C. elastica were introduced in Brazil and occur naturally in northern warm-temperate regions (M. nigra; Berg et al., 2006) and in Central America (F. pertusa and C. elastica; Berg, 2001).
Information about the sampled species of Moraceae. Species are ranked according to their position in the phylogeny of Zerega and Gardner (2019). The order in which species appear in this table follows the cladogram of Zerega and Gardner (2019) (see Fig. 2).
| Species . | Tribe . | Place of collection . | Number of collector . | Date of collection . |
|---|---|---|---|---|
| Maclura tinctoria | Maclureae | Campus USP, Ribeirão Preto, Brazil | V. G. Leite 06 and E. S. Campos (SPFR); V. G. Leite 07 and E. S. Campos (SPFR) | September 2012; February 2013; May 2013 |
| Clarisia ilicifolia | Artocarpeae | Serra do Cavalo, Picuã, Aracruz, Brazil | AP Fontana 7845 (MBLM) | October 2013 |
| Morus nigra | Moreae | Campus USP, Ribeirão Preto, Brazil | V. G. Leite 03 and E. S. Campos (SPFR); V. G. Leite 05 and E. S. Campos (SPFR) | July 2012; February 2013 |
| Brosimum gaudichaudii | Dorstenieae | Sitio Tapera, Sacramento, MG, Brazil | V. G. Leite 01 and V. G. Leite (SPFR) | August 2012; October 2012; June 2013 |
| Castilla elastica | Castilleae | Instituto Agronômico de Campinas, Brazil; Botanical Garden of Rio de Janeiro, Brazil | V. G. Leite 04 (SPFR), Carvalho 122 and J. F. Benedito (IAC); J. C. Gomes s/no. (RB291386); F. F. Moreira s/no. (R. Bl 422383) | October 2012; March 2013; December 2014 |
| Ficus pertusa | Ficeae | Campus USP, Ribeirão Preto, Brazil | R. A. S. Pereira et al. 127 (SPFR 9959) | October 2012; February 2013 |
| Species . | Tribe . | Place of collection . | Number of collector . | Date of collection . |
|---|---|---|---|---|
| Maclura tinctoria | Maclureae | Campus USP, Ribeirão Preto, Brazil | V. G. Leite 06 and E. S. Campos (SPFR); V. G. Leite 07 and E. S. Campos (SPFR) | September 2012; February 2013; May 2013 |
| Clarisia ilicifolia | Artocarpeae | Serra do Cavalo, Picuã, Aracruz, Brazil | AP Fontana 7845 (MBLM) | October 2013 |
| Morus nigra | Moreae | Campus USP, Ribeirão Preto, Brazil | V. G. Leite 03 and E. S. Campos (SPFR); V. G. Leite 05 and E. S. Campos (SPFR) | July 2012; February 2013 |
| Brosimum gaudichaudii | Dorstenieae | Sitio Tapera, Sacramento, MG, Brazil | V. G. Leite 01 and V. G. Leite (SPFR) | August 2012; October 2012; June 2013 |
| Castilla elastica | Castilleae | Instituto Agronômico de Campinas, Brazil; Botanical Garden of Rio de Janeiro, Brazil | V. G. Leite 04 (SPFR), Carvalho 122 and J. F. Benedito (IAC); J. C. Gomes s/no. (RB291386); F. F. Moreira s/no. (R. Bl 422383) | October 2012; March 2013; December 2014 |
| Ficus pertusa | Ficeae | Campus USP, Ribeirão Preto, Brazil | R. A. S. Pereira et al. 127 (SPFR 9959) | October 2012; February 2013 |
IAC, Herbário Fanerogâmico e Criptogâmico do Instituto Agronômico de Campinas; MNHN, Muséum National d’Histoire Naturelle; RB, Herbário do Jardim Botânico do Rio de Janeiro; SPFR, Herbário do Departamento de Biologia da Faculdade de Filosofia Ciências e Letras de Ribeirão Preto da Universidade de São Paulo.
Information about the sampled species of Moraceae. Species are ranked according to their position in the phylogeny of Zerega and Gardner (2019). The order in which species appear in this table follows the cladogram of Zerega and Gardner (2019) (see Fig. 2).
| Species . | Tribe . | Place of collection . | Number of collector . | Date of collection . |
|---|---|---|---|---|
| Maclura tinctoria | Maclureae | Campus USP, Ribeirão Preto, Brazil | V. G. Leite 06 and E. S. Campos (SPFR); V. G. Leite 07 and E. S. Campos (SPFR) | September 2012; February 2013; May 2013 |
| Clarisia ilicifolia | Artocarpeae | Serra do Cavalo, Picuã, Aracruz, Brazil | AP Fontana 7845 (MBLM) | October 2013 |
| Morus nigra | Moreae | Campus USP, Ribeirão Preto, Brazil | V. G. Leite 03 and E. S. Campos (SPFR); V. G. Leite 05 and E. S. Campos (SPFR) | July 2012; February 2013 |
| Brosimum gaudichaudii | Dorstenieae | Sitio Tapera, Sacramento, MG, Brazil | V. G. Leite 01 and V. G. Leite (SPFR) | August 2012; October 2012; June 2013 |
| Castilla elastica | Castilleae | Instituto Agronômico de Campinas, Brazil; Botanical Garden of Rio de Janeiro, Brazil | V. G. Leite 04 (SPFR), Carvalho 122 and J. F. Benedito (IAC); J. C. Gomes s/no. (RB291386); F. F. Moreira s/no. (R. Bl 422383) | October 2012; March 2013; December 2014 |
| Ficus pertusa | Ficeae | Campus USP, Ribeirão Preto, Brazil | R. A. S. Pereira et al. 127 (SPFR 9959) | October 2012; February 2013 |
| Species . | Tribe . | Place of collection . | Number of collector . | Date of collection . |
|---|---|---|---|---|
| Maclura tinctoria | Maclureae | Campus USP, Ribeirão Preto, Brazil | V. G. Leite 06 and E. S. Campos (SPFR); V. G. Leite 07 and E. S. Campos (SPFR) | September 2012; February 2013; May 2013 |
| Clarisia ilicifolia | Artocarpeae | Serra do Cavalo, Picuã, Aracruz, Brazil | AP Fontana 7845 (MBLM) | October 2013 |
| Morus nigra | Moreae | Campus USP, Ribeirão Preto, Brazil | V. G. Leite 03 and E. S. Campos (SPFR); V. G. Leite 05 and E. S. Campos (SPFR) | July 2012; February 2013 |
| Brosimum gaudichaudii | Dorstenieae | Sitio Tapera, Sacramento, MG, Brazil | V. G. Leite 01 and V. G. Leite (SPFR) | August 2012; October 2012; June 2013 |
| Castilla elastica | Castilleae | Instituto Agronômico de Campinas, Brazil; Botanical Garden of Rio de Janeiro, Brazil | V. G. Leite 04 (SPFR), Carvalho 122 and J. F. Benedito (IAC); J. C. Gomes s/no. (RB291386); F. F. Moreira s/no. (R. Bl 422383) | October 2012; March 2013; December 2014 |
| Ficus pertusa | Ficeae | Campus USP, Ribeirão Preto, Brazil | R. A. S. Pereira et al. 127 (SPFR 9959) | October 2012; February 2013 |
IAC, Herbário Fanerogâmico e Criptogâmico do Instituto Agronômico de Campinas; MNHN, Muséum National d’Histoire Naturelle; RB, Herbário do Jardim Botânico do Rio de Janeiro; SPFR, Herbário do Departamento de Biologia da Faculdade de Filosofia Ciências e Letras de Ribeirão Preto da Universidade de São Paulo.
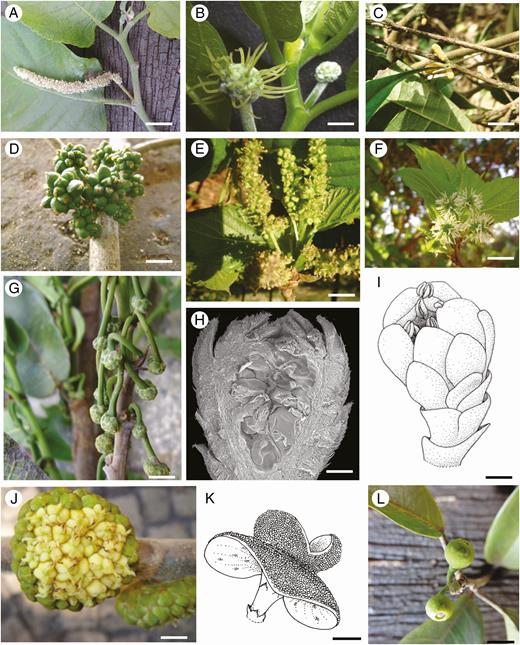
Inflorescence morphology of Moraceae species. (A, B) Staminate (A) and pistillate (B) inflorescences of Maclura tinctoria. (C, D) Staminate (C) and pistillate (D) inflorescences of Clarisia ilicifolia. (E, F) Staminate (E) and pistillate (F) inflorescences of Morus nigra. (G) Inflorescence of Brosimum gaudichaudii. (H, J) Staminate (H, I) and pistillate (J) inflorescences of Castilla elastica (cosexual tree). (K) Complementary staminate inflorescence of C. elastica (male tree). (L) Inflorescence of Ficus pertusa. Scale bars = 10 mm.
About 50–150 inflorescences at different stages of development were collected and analysed for each species. At least two individuals were sampled for each monoecious species and four for the dioecious species. For C. elastica, an androdioecious species, the pistillate inflorescences were collected on a tree producing both pistillate and staminate inflorescences (= cosexual tree) and the staminate inflorescences were collected from a tree producing only staminate inflorescence (= male tree). The plants were georeferenced, and the vouchers were deposited in the MBLM, SPFR and RB herbaria (Table 1).
Samples were fixed in buffered formalin (Lillie, 1954) or Karnovsky’s solution (0.075 mol m−1 in phosphate buffer, pH 7.2–7.4) (Karnovsky, 1965) for 24 h, gradually dehydrated in an ethanol series and stored in 70 % ethanol. They were dissected and prepared for surface analysis by scanning electron microscopy and for anatomical analysis by light microscopy. For scanning electron microscopy, samples were critical-point dried in a Balzers CPD 030 dryer, mounted on aluminium stubs with colloidal carbon, coated with gold in a Bal-Tec SCD 050 sputter coater and observed with Jeol JSM 6610LV and Zeiss EVO 50 scanning electron microscopes. For light microscopy, samples were embedded in historesin (Gerrits and Horobin, 1991) and cut transversely and longitudinally into 2- to 5-μm-thick sections with a rotary microtome (Leica RM2245). Sections were stained with 0.05 % toluidine blue (O’Brien et al., 1964), mounted in synthetic resin (Gerlach, 1969) and observed under a Leica DM5000 B light microscope. Photomicrographs were taken with a Leica DM5000 B microscope coupled to a Leica DFC295 digital camera.
The terminology for bracts was based on Berg (1989), with modifications. The bracts are classified as follows according to the order in which they are initiated during the development of the inflorescence: bract of the inflorescence receptacle (first to arise); interfloral bract (arising among flowers); involucral bract (arising around the receptacle in Castilla and some species of Ficus); orobract (arising at the syconium orifice in Ficus). The interfloral bracts are further classified according to shape as simple or peltate (Ribeiro, 2007).
The terminology for inflorescence type was based on Troll (1928), Weberling (1989) and Endress (2010). The inflorescence is characterized as a raceme if all first- and second-order axes are elongated; as a spike if the first-order axis is elongated and the second-order axis is short; and as a head if all axes are short in the branching region. In this last case, the axis is reduced and can be conical and wide, discoid and plane, or flashy; and in some cases it is surrounded by bracts.
The definitions of breeding systems included in Table 2 follow Ronse De Craene (2010), Sakai (2001) and Richards (1986): a dioecious species exhibits staminate and pistillate flowers on different plants; a monoecious species exhibits staminate and pistillate flowers in the same plant; in an androdioecious species plants with staminate flowers and plants with both staminate and pistillate flowers coexist.
Diagnostic characters of inflorescence morphology in the species studied. The order in which species appear in this table follows the cladogram of Zerega and Gardner (2019) (see Fig. 2)
| Species . | Breeding system/pollinator agent . | Floral composition of inflorescence . | Type of inflorescence . | Number of flowers . | Receptacle shape . | External receptacle bract . | Simple interfloral bract . | Peltate interfloral bract . |
|---|---|---|---|---|---|---|---|---|
| Morus nigra | Dioecious/wind | Staminate | Raceme | 30 (pedicellate) | Elongated | – | – | – |
| Pistillate | Spike | 53 (sessile) | Oval | – | – | – | ||
| Clarisia ilicifolia | Dioecious/gall midge | Staminate | Spike | 150 (sessile) | Elongated | + | + | |
| Pistillate | Head | 15 (sessile) | Globose | + | + | |||
| Maclura tinctoria | Dioecious/wind | Staminate | Spike | 150 (sessile) | Elongated | – | + | – |
| Pistillate | Head | 40 (sessile) | Globose | – | + | – | ||
| Brosimum gaudichaudii | Monoecious/wind | Staminate/pistillate | Head | 10 staminate/1 pistillate | Globose | + | – | + |
| Castilla elastica | Androdioecious/thrips | Staminate (male tree) | Head | 50 (sessile) | Bivalvate | – (imbricate) | – | – |
| Staminate (cosexual tree) | Head | 400 | Urceolate | + (imbricate, interfloral) | – | n/a | ||
| Pistillate (cosexual tree) | Head | 16 (sessile) | Discoid with central depression | + (imbricate) | – | – | ||
| Ficus pertusa | Monoecious/fig wasps | Staminate/pistillate | Syconium | 20 staminate/ 190 pistillate | Urn (closed) | + (orobracts) | n/a | n/a |
| Species . | Breeding system/pollinator agent . | Floral composition of inflorescence . | Type of inflorescence . | Number of flowers . | Receptacle shape . | External receptacle bract . | Simple interfloral bract . | Peltate interfloral bract . |
|---|---|---|---|---|---|---|---|---|
| Morus nigra | Dioecious/wind | Staminate | Raceme | 30 (pedicellate) | Elongated | – | – | – |
| Pistillate | Spike | 53 (sessile) | Oval | – | – | – | ||
| Clarisia ilicifolia | Dioecious/gall midge | Staminate | Spike | 150 (sessile) | Elongated | + | + | |
| Pistillate | Head | 15 (sessile) | Globose | + | + | |||
| Maclura tinctoria | Dioecious/wind | Staminate | Spike | 150 (sessile) | Elongated | – | + | – |
| Pistillate | Head | 40 (sessile) | Globose | – | + | – | ||
| Brosimum gaudichaudii | Monoecious/wind | Staminate/pistillate | Head | 10 staminate/1 pistillate | Globose | + | – | + |
| Castilla elastica | Androdioecious/thrips | Staminate (male tree) | Head | 50 (sessile) | Bivalvate | – (imbricate) | – | – |
| Staminate (cosexual tree) | Head | 400 | Urceolate | + (imbricate, interfloral) | – | n/a | ||
| Pistillate (cosexual tree) | Head | 16 (sessile) | Discoid with central depression | + (imbricate) | – | – | ||
| Ficus pertusa | Monoecious/fig wasps | Staminate/pistillate | Syconium | 20 staminate/ 190 pistillate | Urn (closed) | + (orobracts) | n/a | n/a |
+, presence; –, absence; n/a = information not available.
Diagnostic characters of inflorescence morphology in the species studied. The order in which species appear in this table follows the cladogram of Zerega and Gardner (2019) (see Fig. 2)
| Species . | Breeding system/pollinator agent . | Floral composition of inflorescence . | Type of inflorescence . | Number of flowers . | Receptacle shape . | External receptacle bract . | Simple interfloral bract . | Peltate interfloral bract . |
|---|---|---|---|---|---|---|---|---|
| Morus nigra | Dioecious/wind | Staminate | Raceme | 30 (pedicellate) | Elongated | – | – | – |
| Pistillate | Spike | 53 (sessile) | Oval | – | – | – | ||
| Clarisia ilicifolia | Dioecious/gall midge | Staminate | Spike | 150 (sessile) | Elongated | + | + | |
| Pistillate | Head | 15 (sessile) | Globose | + | + | |||
| Maclura tinctoria | Dioecious/wind | Staminate | Spike | 150 (sessile) | Elongated | – | + | – |
| Pistillate | Head | 40 (sessile) | Globose | – | + | – | ||
| Brosimum gaudichaudii | Monoecious/wind | Staminate/pistillate | Head | 10 staminate/1 pistillate | Globose | + | – | + |
| Castilla elastica | Androdioecious/thrips | Staminate (male tree) | Head | 50 (sessile) | Bivalvate | – (imbricate) | – | – |
| Staminate (cosexual tree) | Head | 400 | Urceolate | + (imbricate, interfloral) | – | n/a | ||
| Pistillate (cosexual tree) | Head | 16 (sessile) | Discoid with central depression | + (imbricate) | – | – | ||
| Ficus pertusa | Monoecious/fig wasps | Staminate/pistillate | Syconium | 20 staminate/ 190 pistillate | Urn (closed) | + (orobracts) | n/a | n/a |
| Species . | Breeding system/pollinator agent . | Floral composition of inflorescence . | Type of inflorescence . | Number of flowers . | Receptacle shape . | External receptacle bract . | Simple interfloral bract . | Peltate interfloral bract . |
|---|---|---|---|---|---|---|---|---|
| Morus nigra | Dioecious/wind | Staminate | Raceme | 30 (pedicellate) | Elongated | – | – | – |
| Pistillate | Spike | 53 (sessile) | Oval | – | – | – | ||
| Clarisia ilicifolia | Dioecious/gall midge | Staminate | Spike | 150 (sessile) | Elongated | + | + | |
| Pistillate | Head | 15 (sessile) | Globose | + | + | |||
| Maclura tinctoria | Dioecious/wind | Staminate | Spike | 150 (sessile) | Elongated | – | + | – |
| Pistillate | Head | 40 (sessile) | Globose | – | + | – | ||
| Brosimum gaudichaudii | Monoecious/wind | Staminate/pistillate | Head | 10 staminate/1 pistillate | Globose | + | – | + |
| Castilla elastica | Androdioecious/thrips | Staminate (male tree) | Head | 50 (sessile) | Bivalvate | – (imbricate) | – | – |
| Staminate (cosexual tree) | Head | 400 | Urceolate | + (imbricate, interfloral) | – | n/a | ||
| Pistillate (cosexual tree) | Head | 16 (sessile) | Discoid with central depression | + (imbricate) | – | – | ||
| Ficus pertusa | Monoecious/fig wasps | Staminate/pistillate | Syconium | 20 staminate/ 190 pistillate | Urn (closed) | + (orobracts) | n/a | n/a |
+, presence; –, absence; n/a = information not available.
RESULTS
The studied species are dioecious, monoecious or androdioecious. The inflorescence varies in terms of type (raceme, spike, head and syconium), format of the receptacle (elongated, oval, discoid, urceolate) and number of flowers per inflorescence (1 to ~190) (Table 2, Fig. 1).
The inflorescence morphogenesis is also highly variable. No species pair followed the same inflorescence morphogenesis (Table 3, Fig. 2). Variation was mainly observed in the shape of the inflorescence meristem (bulging, with a central depression, hemispheric, elongated), in the emergence order and arrangement of flowers along the inflorescence receptacle (Table 3, Fig. 2), and in the occurrence of associated bracts.
Diagnostic characters of inflorescence development in the species studied. The order in which species appear in this table follows the cladogram of Zerega and Gardner (2019) (see Fig. 2)
| Species . | Floral composition of inflorescence . | Inflorescence meristem format . | Number of inflorescence meristems . | Occurrence of vegetative meristem . | Initiation order of flowers along receptacle . | Initiation order of structures in inflorescence meristems . |
|---|---|---|---|---|---|---|
| Morus nigra | Staminate | Elongated | 1 | Yes | Centripetal | Receptacle, floral meristems, peduncle |
| Pistillate | Elongated | 2–3 | Sometimes | Centripetal | ||
| Clarisia ilicifolia | Staminate | Elongated | 1 | Yes | Acropetal | Receptacle, receptacle bracts, floral meristems, peduncle |
| Pistillate | Elongated and elliptical | 1 | Yes | Asynchronous | ||
| Maclura tinctoria | Staminate | Elliptical | 1 | Yes | Acropetal | Receptacle, simple interfloral bracts + floral meristems, peduncle |
| Pistillate | Elliptical | 1 | Yes | Centrifugal | ||
| Brosimum gaudichaudii | Staminate/pistillate | Elliptical | 2 | Yes | Centrifugal (staminate flowers) | Receptacle, receptacle bracts, pistillate floral meristem, peltate interfloral bracts, staminate floral meristems, peduncle |
| Castilla elastica | Staminate (male tree) | Elliptical | 2 | n/a | Asynchronous | Receptacle, receptacle bracts, staminate floral meristems + interstaminal bract |
| Staminate (cosexual tree) | n/a | n/a | n/a | n/a | n/a | |
| Pistillate (cosexual tree) | Elliptical | 1 | n/a | Centrifugal | receptacle, receptacle bracts, pistillate floral meristems | |
| Ficus pertusa | Staminate/pistillate | Elongated | 2 | Yes | Asynchronous | Receptacle, receptacle bracts, orobracts, pistillate and staminate floral meristems + peltate interfloral bracts, peduncle |
| Species . | Floral composition of inflorescence . | Inflorescence meristem format . | Number of inflorescence meristems . | Occurrence of vegetative meristem . | Initiation order of flowers along receptacle . | Initiation order of structures in inflorescence meristems . |
|---|---|---|---|---|---|---|
| Morus nigra | Staminate | Elongated | 1 | Yes | Centripetal | Receptacle, floral meristems, peduncle |
| Pistillate | Elongated | 2–3 | Sometimes | Centripetal | ||
| Clarisia ilicifolia | Staminate | Elongated | 1 | Yes | Acropetal | Receptacle, receptacle bracts, floral meristems, peduncle |
| Pistillate | Elongated and elliptical | 1 | Yes | Asynchronous | ||
| Maclura tinctoria | Staminate | Elliptical | 1 | Yes | Acropetal | Receptacle, simple interfloral bracts + floral meristems, peduncle |
| Pistillate | Elliptical | 1 | Yes | Centrifugal | ||
| Brosimum gaudichaudii | Staminate/pistillate | Elliptical | 2 | Yes | Centrifugal (staminate flowers) | Receptacle, receptacle bracts, pistillate floral meristem, peltate interfloral bracts, staminate floral meristems, peduncle |
| Castilla elastica | Staminate (male tree) | Elliptical | 2 | n/a | Asynchronous | Receptacle, receptacle bracts, staminate floral meristems + interstaminal bract |
| Staminate (cosexual tree) | n/a | n/a | n/a | n/a | n/a | |
| Pistillate (cosexual tree) | Elliptical | 1 | n/a | Centrifugal | receptacle, receptacle bracts, pistillate floral meristems | |
| Ficus pertusa | Staminate/pistillate | Elongated | 2 | Yes | Asynchronous | Receptacle, receptacle bracts, orobracts, pistillate and staminate floral meristems + peltate interfloral bracts, peduncle |
n/a, information not available.
Diagnostic characters of inflorescence development in the species studied. The order in which species appear in this table follows the cladogram of Zerega and Gardner (2019) (see Fig. 2)
| Species . | Floral composition of inflorescence . | Inflorescence meristem format . | Number of inflorescence meristems . | Occurrence of vegetative meristem . | Initiation order of flowers along receptacle . | Initiation order of structures in inflorescence meristems . |
|---|---|---|---|---|---|---|
| Morus nigra | Staminate | Elongated | 1 | Yes | Centripetal | Receptacle, floral meristems, peduncle |
| Pistillate | Elongated | 2–3 | Sometimes | Centripetal | ||
| Clarisia ilicifolia | Staminate | Elongated | 1 | Yes | Acropetal | Receptacle, receptacle bracts, floral meristems, peduncle |
| Pistillate | Elongated and elliptical | 1 | Yes | Asynchronous | ||
| Maclura tinctoria | Staminate | Elliptical | 1 | Yes | Acropetal | Receptacle, simple interfloral bracts + floral meristems, peduncle |
| Pistillate | Elliptical | 1 | Yes | Centrifugal | ||
| Brosimum gaudichaudii | Staminate/pistillate | Elliptical | 2 | Yes | Centrifugal (staminate flowers) | Receptacle, receptacle bracts, pistillate floral meristem, peltate interfloral bracts, staminate floral meristems, peduncle |
| Castilla elastica | Staminate (male tree) | Elliptical | 2 | n/a | Asynchronous | Receptacle, receptacle bracts, staminate floral meristems + interstaminal bract |
| Staminate (cosexual tree) | n/a | n/a | n/a | n/a | n/a | |
| Pistillate (cosexual tree) | Elliptical | 1 | n/a | Centrifugal | receptacle, receptacle bracts, pistillate floral meristems | |
| Ficus pertusa | Staminate/pistillate | Elongated | 2 | Yes | Asynchronous | Receptacle, receptacle bracts, orobracts, pistillate and staminate floral meristems + peltate interfloral bracts, peduncle |
| Species . | Floral composition of inflorescence . | Inflorescence meristem format . | Number of inflorescence meristems . | Occurrence of vegetative meristem . | Initiation order of flowers along receptacle . | Initiation order of structures in inflorescence meristems . |
|---|---|---|---|---|---|---|
| Morus nigra | Staminate | Elongated | 1 | Yes | Centripetal | Receptacle, floral meristems, peduncle |
| Pistillate | Elongated | 2–3 | Sometimes | Centripetal | ||
| Clarisia ilicifolia | Staminate | Elongated | 1 | Yes | Acropetal | Receptacle, receptacle bracts, floral meristems, peduncle |
| Pistillate | Elongated and elliptical | 1 | Yes | Asynchronous | ||
| Maclura tinctoria | Staminate | Elliptical | 1 | Yes | Acropetal | Receptacle, simple interfloral bracts + floral meristems, peduncle |
| Pistillate | Elliptical | 1 | Yes | Centrifugal | ||
| Brosimum gaudichaudii | Staminate/pistillate | Elliptical | 2 | Yes | Centrifugal (staminate flowers) | Receptacle, receptacle bracts, pistillate floral meristem, peltate interfloral bracts, staminate floral meristems, peduncle |
| Castilla elastica | Staminate (male tree) | Elliptical | 2 | n/a | Asynchronous | Receptacle, receptacle bracts, staminate floral meristems + interstaminal bract |
| Staminate (cosexual tree) | n/a | n/a | n/a | n/a | n/a | |
| Pistillate (cosexual tree) | Elliptical | 1 | n/a | Centrifugal | receptacle, receptacle bracts, pistillate floral meristems | |
| Ficus pertusa | Staminate/pistillate | Elongated | 2 | Yes | Asynchronous | Receptacle, receptacle bracts, orobracts, pistillate and staminate floral meristems + peltate interfloral bracts, peduncle |
n/a, information not available.
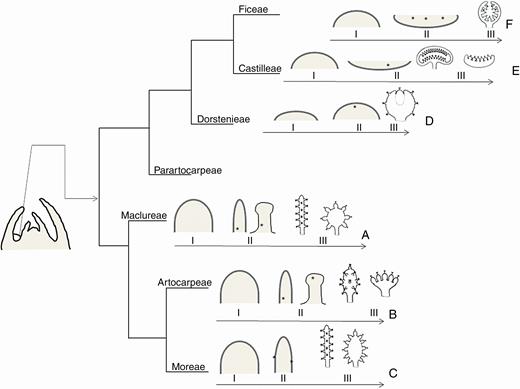
Schematic drawings summarizing information about three developmental stages of the inflorescence, plotted in a recent cladogram of Moraceae (Zerega and Gardner, 2019). Each plotted species represents a tribe. (A) Maclura tinctoria. I, elliptic meristem; II, meristem forms a horizontally elongated receptacle at whose margins floral meristems arise from the base to the apex; III, receptacle acquires an elongated (staminate) or globose (pistillate) format. (B) Clarisia ilicifolia. I, elongated meristem; II, meristem forms a horizontally elongated receptacle at whose margins floral meristems arise from the base to the apex; III, receptacle acquires an elongated (staminate) or globose (pistillate) shape. (C) Morus nigra. I, meristem elongates vertically; II, meristem forms a vertically elongated receptacle at whose lateral sides flower meristems arise; III, receptacle acquires an elongated (staminate) or oval (pistillate) format. (D) Brosimum gaudichaudii. I, flat meristem; II, meristem forms a spherical receptacle where the central floral meristems emerge; III, receptacle with immersed flowers. (E) Castilla elastica. I, elliptic meristem; II, meristem forms a concave receptacle in whose central depression floral meristems emerge; III, the margins of the receptacle do not close and the receptacle acquires a bivalvate (staminate, male tree) or discoid (pistillate, cosexual tree) format. (F) Ficus pertusa. I, hemispheric meristem; II, meristem forms a bulging receptacle where the first floral meristems emerge; III, the margins of the receptacle expand and enclose the inflorescence. *Floral meristem; I, early stages, meristem shape; II, intermediate stages, location of the first floral meristems; III, late stages, final shape of the inflorescence with flowers attached to a receptacle.
Organography and inflorescence morphogenesis may vary within species between staminate and pistillate inflorescences, as in Maclura tinctoria and Morus nigra (Tables 2 and 3).
Maclura tinctoria, Clarisia ilicifolia and Morus nigra
Organography (Table 2). The species of this subclade are dioecious and have raceme, spike or head types of inflorescences (Fig. 1A–F), 30–150 sessile or pedicellate staminate flowers and 15–50 sessile or pedicellate pistillate flowers. Receptacle bracts are occasionally found and interfloral bracts are generally found.
Inflorescence development (Table 3). One to three inflorescence meristems can be formed in a bract axil, next to or not next to a vegetative meristem. The inflorescence meristem is in general elliptical (Fig. 3). Its distal portion forms a horizontally elongated receptacle where the floral meristems first arise on the lateral sides (and/or adaxial side), from the base to the apex (Fig. 4). In some cases, the distal portion of the inflorescence meristem forms bracts before the emergence of floral meristems (Fig. 4). After the emergence of floral meristems and/or bracts, the proximal portion of the inflorescence meristem elongates and forms a peduncle that can be small or large (Figs 4 and 5). The developing flowers can form or not form a pedicel (Fig. 5).
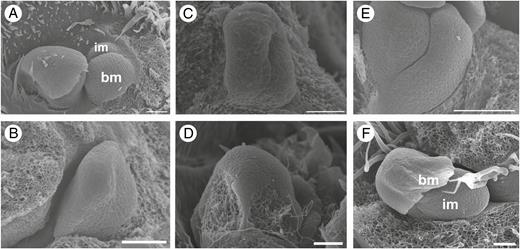
Early developmental stages of inflorescence of Maclura tinctoria (A, B), Clarisia ilicifolia (C, D) and Morus nigra (E, F). (A) Inflorescence meristem in the axil of a subtending bract. (B) Elongation of a pistillate inflorescence meristem forming the receptacle. (C) Elongated staminate inflorescence meristem. Bract removed. (D) Slightly elliptical receptacle (pistillate inflorescence). (E) Staminate inflorescence meristem vertically expanded (bract removed). (F) Pistillate inflorescence meristem in the axil of a bract. bm, bract meristem; im, inflorescence meristem. Scale bars: (A) = 10 μm; (B) = 25 μm: (C, D, F) = 50 μm; (E) = 200 μm.
Intermediate developmental stages of inflorescence of Maclura tinctoria (A, B), Clarisia ilicifolia (C, D) and Morus nigra (E, F). (A) Asynchronous initiation of floral staminate meristems (ellipse). (B) Asynchronous initiation of several floral pistillate meristems (asterisks). (C) Receptacle of staminate inflorescence with asynchronously emerging simple or peltate interfloral bracts (asterisks). (D) Inflorescence with several developing pistillate flowers (asterisks). (E) Initiation of floral staminate meristems on the sides of the receptacle (asterisks). (F) Initiation of floral pistillate meristems on the lateral surface of the receptacle (asterisks). Scale bars: (A) = 200 μm; (B) = 50 μm; (C–F) = 100 μm.
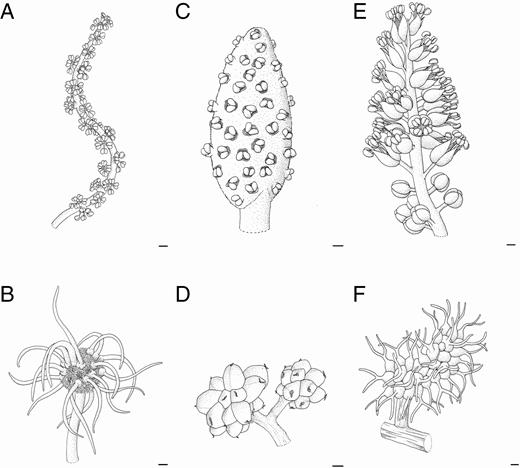
Final developmental stages of inflorescence of Maclura tinctoria (A, B), Clarisia ilicifolia (C, D) and Morus nigra (E, F). (A) General inflorescence scheme. Note the elongated receptacle and the arrangement of staminate flowers. (B) General inflorescence scheme. Note the globose shape of the inflorescence receptacle with pistillate flowers. (C) General inflorescence scheme. Note the staminate flowers arranged along the inflorescence receptacle. (D) General inflorescence scheme. Note the rounded receptacle with pistillate flowers in late developmental stages. (E) General inflorescence scheme. Note the staminate flowers arranged along an elongated receptacle. (F) General inflorescence scheme with pistillate flowers arranged along the receptacle. Scale bars: (A–F) = 200 μm.
Brosimum gaudichaudii, Castilla elastica and Ficus pertusa
Organography (Table 2). The species of this subclade are monoecious or androdioecious and have raceme or syconium types of inflorescences (Fig. 1G–L), 9–150 pedicellate staminate flowers and 1–190 pedicellate/sessile pistillate flowers. Receptacle bracts are always found and interfloral bracts do not occur in general.
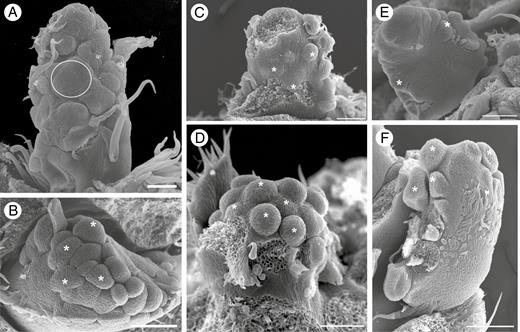
Inflorescence development (Table 3). The inflorescence meristem is rounded and subtended by a bract (Fig. 6). Its distal portion forms a concave receptacle where bracts arise, covering the developing inflorescence (Fig. 7). Next, the receptacle thickens, remaining flat (B. gaudichaudii) or forms a central depression with margins largely expanded (Fig. 7). The margins of the receptacle do not close and the receptacle acquires a bivalvate or discoid format (C. elastica) or the margins completely enclose the inflorescence resulting in an urceolate form (F. pertusa) (Figs 7 and 8; Supplementary Data Fig. S1). The floral meristems emerge in the centre of the receptacle or asynchronously (Fig. 7) and the proximal portion of the inflorescence meristem forms a peduncle (Fig. 8).
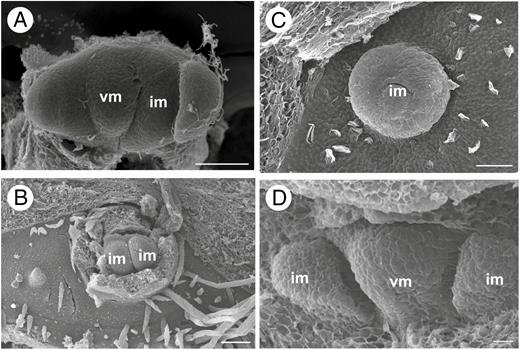
Early developmental stages of inflorescence of Brosimum gaudichaudii (A), Castilla elastica (B, C) and Ficus pertusa (D). (A) Triad of meristems with two lateral inflorescence meristems and a central vegetative meristem. (B) Elliptical receptacle of staminate inflorescence. Bracts removed. (C) Elliptical receptacle of pistillate inflorescence (bract removed). (D) Triad of meristems with two lateral inflorescence meristems and a central vegetative meristem. im, inflorescence meristem; vm, vegetative meristem. Scale bars (A, B) = 100 μm; (C) = 50 μm; (D) = 10 μm.
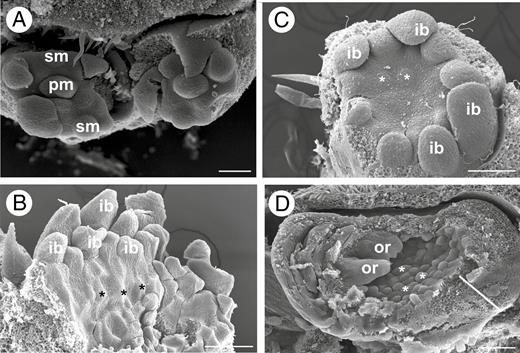
Intermediate developmental stages of inflorescence of Brosimum gaudichaudii (A), Castilla elastica (B, C) and Ficus pertusa (D). (A) Frontal view of the inflorescence. Note the initiation of a pistillate and staminate flower meristem. (B) Emergence of first floral staminate meristems along the receptacle (asterisks). (C) Initiation of the first floral pistillate meristem in the receptacle (asterisks). (D) Expansion of a lateral inflorescence meristem that assumes a concave shape. Note the emergence of floral meristems (asterisks) and orobracts, and the enlargement of the inflorescence wall white line). ib, involucral bract; or, orobracts; pm, pistillate flower primordium; sm, staminate flower primordium. Scale bars (A–D) = 100 μm.
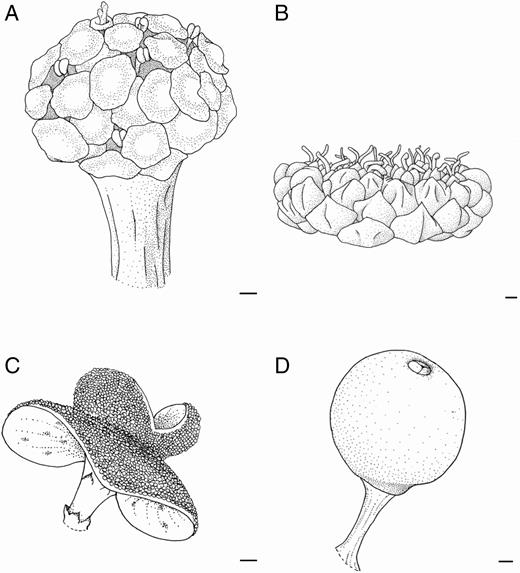
Final developmental stages of inflorescence of Brosimum gaudichaudii (A), Castilla elastica (B, C) and Ficus pertusa (D). (A) General inflorescence appearance. Note the peltate bracts covering the staminate and pistillate flowers. (B) General inflorescence appearance. Note the bivalvate receptacle completely open and stamens exposed. (C) General inflorescence appearance. Note the involucral bracts surrounding the receptacle. (D) General inflorescence appearance. Scale bars (A–D) = 200 μm.
DISCUSSION
We present data for two monoecious species whose two types of floral morph (staminate and pistillate) are found in the same inflorescence (B. gaudichaudii and F. pertusa), data for three dioecious species with floral morph types organized into two types of inflorescence (staminate and pistillate) very different from each other (C. ilicifolia, M. tinctoria, M. nigra), and data for an androdioecious species in which one type of plant presents the two types of flower in separate inflorescences (staminate and pistillate) and the other type of plant only presents inflorescences with staminate flowers. In the three dioecious species, staminate and pistillate inflorescences are very different from each other (Table 2), possibly due to their functions in the release (staminate) and reception (pistillate) of pollen grains and, for insect-pollinated species, in the attraction and/or breeding of the pollinators. In the case of C. elastica, the androdioecious species, there is variation in receptacle shape and in the occurrence of bracts between the staminate inflorescences found on the two tree types (Table 2). This means that the function of inflorescences containing staminate flowers on “cosexual” plants differs from that of inflorescences on “male” trees. We elaborate the hypothesis that they may serve to attract insects to plants bearing pistillate inflorescences rather than serving for pollinator breeding and pollen dispersal.
What makes a fig? Transitions in the inflorescence structure of Moraceae species
Among the species studied here, B. gaudichaudii and C. elastica belong to clades closely related to the tribe of F. pertusa (Fig. 12 in Clement and Weiblen, 2009; Zerega and Gardner, 2019; Clement et al., 2020). In these three species, inflorescences begin their development as flat and open structures (present study), suggesting that selective pressure has been exerted by pollinators or by herbivores and seed predators to enclose the flowers inside the inflorescence, culminating in the syconium of Ficus. The urn-shaped receptacle of Ficus is closed at the apex by orobracts forming a narrow passage (ostiole) so that the numerous internally distributed diclinous flowers can only be pollinated by fig wasps and pollen can only be collected from a syconium by the wasps born inside it (Hymenoptera, Agaonidae), characterizing an obligatory mutualism between fig trees and fig wasps (Moe et al., 2012). The orobracts serve at least two functions in Ficus: enclosing the syconium and emitting receptive syconium odours that will attract pollinators (Souza et al., 2015; Hu et al., 2020). In some species, epidermal cells of ostiolar bracts produce an exudate that plugs the entrance of the syconium after pollinator entry (Machado et al., 2013). We propose here that they perform one more function, moulding the development of the flowers by filling up the future syconium cavity, thus controlling flower development, as suggested for Ficus sur (Verkerke, 1988).
An evident transitional stage of this enclosure is observed in the three different types of inflorescence of C. elastica: (1) discoid head, condensed inflorescence with a receptacle almost totally enclosed by marginal involucral bracts and united flowers (pistillate inflorescence); (2) urceolate ‘fig-like’ head inflorescence with an apical orifice covered with imbricate bracts (complementary staminate inflorescences found in ‘cosex’ trees); and (3) bivalvate inflorescence enclosed by marginal bracts (staminate inflorescences found in male trees). These forms of inflorescence do not completely enclose the flowers, but, even so, staminate inflorescences only expose the staminate flowers late during inflorescence development. Castilla is pollinated by thrips (Sakai, 2001) and, as proposed for Ficus, we hypothesize that pistillate flowers are not the main structures involved in pollinator attraction, but staminate inflorescences on cosexual plants could also play that role.
In B. gaudichaudii (Jacomassi et al., 2010; present study) and some Dorstenia species (Araújo et al., 2017), the inflorescence receptacle is expanded and thick but does not enclose pistillate or staminate flowers. However, these flowers are almost totally immersed in the receptacle and thus are hidden in the inflorescence. Little is known about pollination in the tribe Dorstenieae, which includes Brosimum and Dorstenia (Berg et al., 2006). Brosimum alicastrum is anemophilous, but it could be atypical in this respect within the genus (Berg, 2001). Dorstenia has been better studied. Beetles (Hoen and Punt, 1989; Berg and Hijman, 1999) and flies (Berg and Hijman, 1999; Araújo et al., 2017) were found visiting the flowers immersed in the cup- or flask-shaped receptacle of the cenanthum (Golenkin, 1894). An important aspect here is the strong protogyny within the inflorescence. The female phase may last 14–15 d and the male phase may last over 30 d with limited, if any, overlap (Araújo et al., 2017). Hence, the offspring of an insect ovipositing during the female phase may emerge when the inflorescence is still producing pollen. This phenology was probably a prerequisite for the occurrence of the mutualistic pollination system between Ficus and Agaonid wasps.
The enclosure and protection of flowers by receptacles whose margins elongate and completely or partially enclose the inflorescence have several consequences for the pollination of this entomophilous group of plants. Pollinating insects (flies in Dorstenia arifolia, thrips in C. elastica and fig wasps in species of F. pertusa and other species) are attracted to the inflorescence by odour glands (Souza et al., 2015) located externally on the receptacle and in the various types of bracts found at the inflorescence closure sites, such as the ostiole in Ficus (Machado et al., 2013; Souza et al., 2015). Glands secreting nectar (extrafloral nectary), mucilage (colleters), phenolic compounds (idioblasts or secretory epidermis) and latex (laticifers) can also be found (Machado et al., 2013; Souza et al., 2015; Marinho and Teixeira, 2019) on both surfaces of the receptacle, with functions concerning the protection of the inflorescence against radiation and herbivores, lubrication of the developing floral organs and short-distance attraction of pollinators. These insects use inflorescences for oviposition and offspring protection (‘nursery pollination’) and even for mating, while transferring pollen passively (C. elastica; Zerega et al., 2004) or actively (F. pertusa; Frank, 1984; Kjellberg et al., 2001) between flowers.
In addition, these inflorescences acquire the appearance of a floral unit (Berg, 1972), i.e. a pseudanthous inflorescence. Pseudanthy is correlated with the discoid and urceolate shapes of receptacles, especially if they are involucrate or bear marginal bracts (e.g. Castilleae and Dorstenieae; Berg, 1977). Reduction of such inflorescences can also result in uniflorous pistillate inflorescences, as is the case for Ficus oleifolia and Perebea humilis (Berg, 1977; Berg et al., 2006).
Racemes, spikes and heads of Maclura tinctoria, Clarisia ilicifolia and Morus nigra
Unlike B. gaudichaudii, C. elastica and F. pertusa, the inflorescences of C. ilicifolia, M. tinctoria and M. nigra are open, characterized as elongated spikes, globose heads or oval racemes (Table 2). The raceme characterizes the pistillate inflorescence of M. nigra while the short floral pedicels characterize the pistillate inflorescence of M. nigra and staminate inflorescences of C. ilicifolia and M. tinctoria as spikes. An inflorescence is characterized as a raceme if all first- and second-order axes are elongated, as a spike if the first-order axis is elongated and the second axis is short, and as a head if all axes are short in the branching region. In the last case the axis is reduced and can be conical and wide, or even discoid (Table 2) and plane, and can be surrounded by bracts (Troll, 1928; Weberling, 1989; Endress, 2010). Although the inflorescence meristem is shaped similarly in C. ilicifolia, M. tinctoria and M. nigra, the order of flower initiation along the receptacle is highly variable (acropetal, asynchronous, centrifugal; Table 2) even in the same species, when we consider pistillate and staminate flowers (e.g. C. ilicifolia).
Morus nigra and Maclura tinctoria are two wind-pollinated species having inflexed filaments and anthers fitted in the sepals, which is associated with an explosive mechanism of pollen release (Pedersoli et al., 2019). This mechanism allows the release of pollen grains into the air at initial velocities exceeding Mach 0.7 (Taylor et al., 2006). We show that the ontogeny of the inflorescences differs between the two species for both staminate and pistillate inflorescences. The difference in staminate inflorescence ontogeny suggests functional convergence rather than identity by descent, in agreement with molecular results. Similarly, C. elastica is pollinated by thrips (Sakai, 2001), as is Antiaropsis decipiens (Zerega et al., 2004) within the Castilleae. Clarisia ilicifolia is probably pollinated by insects, as demonstrated for Artocarpus integer (Sakai et al., 2000) and Artocarpus heterophyllus (Gardner et al., 2018) within the tribe Artocarpeae. The development of staminate and pistillate inflorescences is strikingly different between Castilla and Clarisia. However, the analogy in pollination mode may be less marked in this comparison than in the case of Morus and Maclura. It is because in Artocarpeae the pollinating gall midges feed on fungi developing on staminate inflorescences, explaining the broad staminate inflorescence, while in Castilleae the pollinating thrips feed on pollen, explaining the higher density of anthers in its staminate inflorescence (Sakai et al., 2000; Sakai, 2001; Zerega et al., 2004; Gardner et al., 2018).
Another particularly important variable characteristic is related to the arrangement of the inflorescences along the shoot, which may be related to the presence of a vegetative meristem along with the reproductive meristem. This vegetative meristem can systematically produce a leafy shoot with short internodes, as in Maclura tinctoria and Morus nigra, or can sometimes continue its development into a leafy shoot, as in B. gaudichaudii and F. pertusa. In some species of Ficus, such as F. carica (Valdeyron, 1967), or most species of the Pharmacosycea section (F. Kjellberg, Université de Montpellier, CNRS, Montpellier, France, pers. obs.), a single inflorescence meristem (but sometimes two) is generally formed in association with a vegetative meristem. The presence of two syconia is the more general condition in Ficus. In a number of species belonging to diversified subgenera and sections, the vegetative meristem produces a reproductive branch that will bear many figs, with two or more at each internode. The transition from axillated figs to cauliflory has occurred many times, suggesting a simple ontogenic transition. In F. pertusa, however, we observe two syconia (Fig. 1L) from the two inflorescence meristems, and the vegetative meristem centrally positioned in a triad of apices generally aborts (Fig. 1B–F, H). Ontogenic studies on inflorescence in cauliflorous Ficus species would permit confirmation of the hypothesis of a direct association between this vegetative meristem and the production of cauliflorous branches.
Importance of bracts and peduncle for inflorescence architecture of Moraceae
Our data show that bracts, whether of first or second order, peltate or simple, external or interfloral, originate in the inflorescence meristem and are therefore homologous in origin. The functions attributed to the bracts are also numerous. Bracts are cited as a protective organ either of the floral meristem or of the inflorescence meristem (Berg, 1972, 2001; Clement and Weiblen, 2009; present study). In Ficus, the orobracts that line the ostiole play important roles regarding the closure of the syconium (Berg, 1972, 2001; Mello-Filho et al., 2001), the attraction of pollinating fig wasps by compounds secreted by glands (Machado et al., 2013; Souza et al., 2015), and even the order of flower initiation in the syconium’s receptacle because of the pressure they exert on the meristematic tissues (Verkerke, 1988; Basso-Alves et al., 2014; present study). In Maclura, interfloral bracts are covered with globular or elliptical glands with a yellow content, suggesting a defence function against phytophagous insects (Berg, 2001). In F. pertusa and B. gaudichaudii, species that share the presence of a triad of meristems (a vegetative meristem among two reproductive ones), first-order external marginal bracts are observed only during the early development of inflorescences, when there is no contact between meristems in the triad, also indicating a protective function. The interfloral bracts of Moraceae may act as a barrier against phytophagous invasion (Berg, 1990), as illustrated in B. gaudichaudii and in C. ilicifolia (pistillate inflorescence), in which the apices of the peltate bracts are imbricated. In contrast, the simple interfloral bracts have thin apices partially covering the floral meristems in C. ilicifolia (staminate inflorescence) and M. tinctoria (staminate and pistillate inflorescences).
The presence and development of the inflorescence peduncle are already noticeable during the intermediate stages of inflorescence development in most of the studied species of Moraceae (Moncur, 1985; present study). The presence of a peduncle ensures that the inflorescences remain evident in relation to the branches with leaves for a long period of time, thus promoting attractiveness for pollinators (Marshall, 1995). Maclura tinctoria and Morus nigra show elongated, flexible and prominent staminate inflorescences exposed to airflow and susceptible to agitation, which facilitates the removal of pollen, since these species are wind-pollinated. The discoid and wide inflorescence of C. elastica is totally exposed in the branches when the flowers are receptive, since the leaves are deciduous.
Conclusions
Two general patterns of inflorescence development were observed in the study species. In B. gaudichaudii, C. elastica and F. pertusa (subclade that encompasses the tribes Dorstenieae, Castilleae and Ficeae), the inflorescence receptacle is initially flat-discoid and ends in an urceolate inflorescence that encloses the flowers. The inflorescence of Maclura tinctoria, Morus nigra and C. ilicifolia (subclade formed by the tribes Moreae, Maclureae and Artocapeae), on the other hand, begins its development as an elongated structure that results in a spike, a globose head or an oval raceme.
Inflorescence morphogenesis is highly variable in Moraceae. No species pair followed the same inflorescence morphogenesis, even in cases of a similar pollination mode. Such a great diversity of the inflorescence architecture remains a field of research to be explored. The main gap is still the comprehensive understanding of the structure and function of these inflorescences in the family, which could be filled by studies that relate morphological and ecological aspects to inflorescence development.
SUPPLEMENTARY DATA
Supplementary data are available online at https://dbpia.nl.go.kr/aob and consist of the following. Figure S1: aspects of inflorescence development in Ficus pertusa.
FUNDING
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (grant numbers 2012/1544-8 and 2014/07453-3), Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant numbers 305793/2018-7 and 302806/2019-9) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (code number 001).
ACKNOWLEDGEMENTS
We are grateful to Rodrigo Ferreira Silva (FFCLRP-USP), Maria Dolores Seabra Ferreira, José Augusto Maulin (FMRP-USP), Edimárcio da Silva Campos (FCFRP/USP), Juliana Villela Paulino (UFRJ) and André Paviotti Fontana for technical assistance, to Bruno Garcia Simões Favaretto for the drawings (Figs 1I, 1K, 2III, 5A-F, 8A-D) and to Elettra Greene and Dewey Litwiller for English revision. The authors declare that the research was conducted in the absence of any conflict of interest.



