-
PDF
- Split View
-
Views
-
Cite
Cite
Tia-Lynn Ashman, Conchita Alonso, Victor Parra-Tabla, Gerardo Arceo-Gómez, Pollen on stigmas as proxies of pollinator competition and facilitation: complexities, caveats and future directions, Annals of Botany, Volume 125, Issue 7, 4 June 2020, Pages 1003–1012, https://doi.org/10.1093/aob/mcaa012
Close - Share Icon Share
Abstract
Pollen transfer via animals is necessary for reproduction by ~80 % of flowering plants, and most of these plants live in multispecies communities where they can share pollinators. While diffuse plant–pollinator interactions are increasingly recognized as the rule rather than the exception, their fitness consequences cannot be deduced from flower visitation alone, so other proxies, functionally closer to seed production and amenable for use in a broad variety of diverse communities, are necessary.
We conceptually summarize how the study of pollen on stigmas of spent flowers can reflect key drivers and functional aspects of the plant–pollinator interaction (e.g. competition, facilitation or commensalism). We critically evaluate how variable visitation rates and other factors (pollinator pool and floral avoidance) can give rise to different relationships between heterospecific pollen and (1) conspecific pollen on the stigma and (2) conspecific tubes/grain in the style, revealing the complexity of potential interpretations. We advise on best practices for using these proxies, noting the assumptions and caveats involved in their use, and explicate what additional data are required to verify interpretation of given patterns.
We conclude that characterizing pollen on stigmas of spent flowers provides an attainable indirect measure of pollination interactions, but given the complex processes of pollen transfer that generate patterns of conspecific–heterospecific pollen on stigmas these cannot alone determine whether competition or facilitation are the underlying drivers. Thus, functional tests are also needed to validate these hypotheses.
INTRODUCTION
Approximately 80 % of flowering plant species depend on animal pollinators for sexual reproduction (Ollerton et al., 2011). The sufficiency of pollination for a given species, however, can be strongly affected by the abundance and diversity of other plant species in the community (co-flowering effects; Pauw, 2013; Thomson et al., 2019). Thus, understanding how pollinator-mediated interactions between plants affect plant reproduction has been an important pursuit for many decades (reviewed in Knight et al., 2018). It is even more urgent now, given the accelerated rate of anthropogenic threats to this crucial ecosystem service (e.g. land use and climate change; IPBES, 2016).
Pollinator-mediated plant–plant interactions not only affect plant fitness and population growth (e.g. Ashman et al., 2004) but can have implications for the evolution of floral traits (e.g. Caruso, 2000) and for the structure and assembly of plant communities (e.g. Sargent and Ackerly, 2008). Interactions between plant species for pollination services have been described as positive (facilitation), negative (competition) or neutral (commensalism) for one or both of the interacting plant species (reviewed in Braun and Lortie, 2019). While these interactions have been broadly demonstrated in pairs or in a few interacting plant species (e.g. Moeller, 2004; Ghazoul, 2006), the complexity of interactions represented in multispecies communities has necessitated a shift in emphasis away from focusing on pairwise interactions to the more diffuse ones found in most natural communities (e.g. Bascompte et al., 2003).
One approach has been to characterize plant–pollinator networks based on visitation patterns, and these have revealed, among other things, extensive pollinator sharing among plants and high levels of plant and pollinator generalism (e.g. Bascompte et al., 2003). However, visitation networks are limited in that they do not characterize the direction (positive or negative) of plant–plant interactions occurring, nor can they evaluate the full fitness consequences of these interactions (King et al., 2013; Bennett et al., 2018). Thus, attention has shifted away from solely observing pollinator visitation to recording community-level outcomes of the pollination process that take place between flower visitation and seed production (Fig. 1A). Specifically, researchers have begun to record the number and identity of conspecific pollen (CP) and heterospecific pollen (HP) grains transferred to stigmas for multiple species in a community (e.g. Fang and Huang, 2013; King et al., 2013; Emer et al., 2015; Tur et al., 2016; Johnson and Ashman, 2019). Patterns of pollen depostion on stigmas can be powerful in that they convey information on the strength of plant–plant interactions that result from pollinator sharing (e.g. Tur et al., 2016; Thomson et al., 2019), as well as serving as reliable proxies of the fitness consequences of these interactions (CP tube number and seed production; Fig. 1A, B). This is particularly true when the relationship between CP and HP grains and conspecific pollen tubes in the style is further evaluated as it affects the probability of ovule fertilization (Fig. 1B; Aizen and Harder, 2007; Alonso et al., 2012; Arceo-Gómez and Ashman 2014b; Tur et al., 2016). This shift in focus reflects the growing recognition that interacting communities of pollen grains on stigmas can affect post-pollination processes and overall plant fitness (Ashman and Arceo-Gómez, 2013).
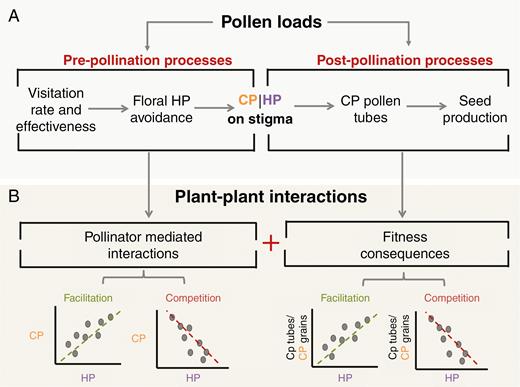
Schematic representation of the pre- and post-pollination processes in the pathway from pollen transport to seed production (A) and how these processes translate into patterns of conspecific pollen (CP) versus heterospecific pollen (HP) on the stigma or patterns of HP and CP/tubes per grain in the style (B). (A) Pre-pollination processes affect CP and HP received by flowers, whereas post-pollination interactions between CP and HP on the stigma affect the number of CP tubes or seeds produced. (B) Patterns between CP and HP grains received, and between CP tubes/grain and HP received per stigma, can reflect pollinator-mediated interactions and their fitness consequences, respectively. Such patterns have been used to infer competitive or facilitative plant–plant interactions. The focus of this paper is to clarify the diversity of drivers that can underlie these patterns (Fig. 2).
Recently, post-pollination patterns of CP and HP receipt have been used as proxies of the strength and direction of pollinator-mediated competitive and facilitative plant–plant interactions in an entire community (Fig. 1B; Tur et al., 2016). This was motivated by the challenges presented by experimental studies of multispecies interactions (e.g. Ghazoul, 2006) and the complex processes influencing pollen transfer (e.g. pollinator diversity, efficiency, visitation rate, floral density and morphology) in multispecies communities (e.g. Minnaar et al., 2018; Albor et al., 2019; Thomson et al., 2019). In particular, the patterns of covariation between CP and HP on stigmas have been used as proxies for pollinator-mediated competition (negative relationship) and facilitation (positive relationship; Fig. 1B) among plant species at the pre-pollination stage (i.e. before pollen deposition on stigmas; Tur et al., 2016). Furthermore, the likelihood of conspecific pollen tube growth as a function of CP and HP receipt (Fig. 1B) has been used in a similar manner to describe plant–plant interactions at the post-pollination stage (i.e. after pollen deposition on stigmas; Alonso et al., 2013; Tur et al., 2016). Evaluating the relationship between HP and CP tubes/grain is particularly important as it more directly informs the fitness effects of HP on the recipient species (reviewed in Morales and Traveset, 2008). This, in turn, better informs the potential for overall facilitative or competitive plant–plant interactions, as the final outcome will strongly depend on the extent of the cumulative effects of HP transfer (i.e. effects via floral visitation and HP interference on the stigma), which have been shown to be highly variable (Ashman and Arceo-Gómez, 2013). Overall, these studies have revealed that there is a wealth of information to be gained from analysis of stigmatic pollen loads collected from natural communities.
The difficulty of conducting manipulative studies to evaluate the strength and direction of community-level plant–plant interactions (e.g. Ghazoul, 2006; Thomson et al., 2019) further suggests that patterns of pollen receipt gathered from stigmas will continue to be an important tool to evaluate ecological interactions among multiple plant species within contemporary communities and as references for understanding how these change over time (Johnson et al., 2019). However, we argue that the lack of acknowledgement of the myriad factors (e.g. relative flower abundance, flower morphology, pollinator visitation rate and diversity) that influence these proxies limits the conclusions that can be made regarding the types of diffuse plant–plant interactions (e.g. competition and facilitation) occurring pre-pollination in natural communities. Thus, a clear framework to study and interpret patterns of covariation between HP receipt, CP receipt, and CP tube growth is urgently needed if we aim to continue using these as proxies of ecological interactions within communities.
Our aim with this viewpoint is to stimulate awareness by discussing caveats, limitations and assumptions of using patterns of pollen receipt and pollen tubes in styles to interpret pollinator-mediated facilitative and competitive processes. We do so by explicating a few examples of how multiple processes can produce similar CP–HP patterns and vice versa and how multiple CP–HP patterns can result from the same pre-pollination processes (e.g. competition). We hope to shed light on the complexity, but also provide some guidelines on how to better evaluate and interpret data on stigmatic pollen loads in a way that helps move this growing field forward. It is also worth acknowledging for clarity that here we exclusively focus on interpreting these patterns from a female fitness perspective because recent studies have only considered this aspect (e.g. Tur et al., 2016). However, male fitness consequences of interspecific pollen transfer (i.e. CP loss) are important contributors to total plant fitness (Muchhala and Thomson, 2012; Moreira-Hernandez and Muchhala, 2019) and these would need to be eventually integrated at the community level to gain a full understanding of plant–plant interactions from pollen loads on stigmas.
Here we focus on key drivers of the associations (1) between CP and HP receipt, and (2) between the proportion of CP tubes/grain and HP receipt (Fig. 1B). To illustrate our arguments we conceptually explore how flower visitation rate (as affected by flower density), floral morphology and features of local pollinator pools could combine to generate the varied linear and non-linear relationships observed in natural communities (e.g. Arceo-Gómez et al., 2016; Tur et al., 2016), although we recognize that other factors may also be important (Minnaar et al., 2018). This heuristic approach reveals that a more careful examination of the variation in pollen loads on stigmas is required to distinguish between pollinator-mediated competition and facilitation. In addition, our analysis indicates that supplementary information will be required to tease apart dominant underlying drivers of variation in pollen loads on stigmas. Nevertheless, stigmatic proxies are an important component of a research programme to understand plant–plant–pollinator interactions.
PROCESSES THAT UNDERLIE CP–HP RELATIONSHIPS
We consider how pollinator visitation rate, pollinator pool diversity/efficiency and floral HP avoidance morphology can contribute to the variation in transfer of pollen to stigmas. We then evaluate the ways these could combine to create a variety of observed patterns among flowers in CP and HP received (Fig. 2). In doing so we illustrate the complexity in interpretation of these patterns and reveal the features that need to be confirmed independently to confidently ascribe underlying processes.
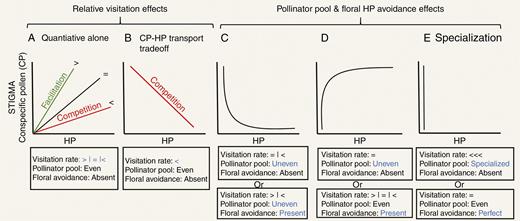
Patterns of conspecific pollen (CP) versus heterospecific pollen (HP) receipt that can be observed on stigmas of spent flowers and potential drivers of variation in these patterns. Potential drivers include variation in pollinator visitation (A, B) or variation in the pollinator pool and floral morphology (C–E). (A, B) Patterns that result from variation in quantity of pollinator visits. In (A), when visitation rate for the focal species is higher (>) or lower (<) in the presence of coflowering species, the pattern can reflect facilitation (green) or competition (red), respectively. However, in (B) lower visitation rate is accompanied by a pollen-transport trade-off leading to a negative CP–HP relationship. (C–E) CP–HP relationships when variation in pollinator pool and floral avoidance mechanisms exist regardless of visitation differences (blue for emphasis of mechanism). (C) Uneven pollinator pools combined with or without floral avoidance can lead to non-linear negative CP–HP relationships. (D) Uneven pollinator pools or presence of floral avoidance can lead to non-linear positive CP–HP relationships. (E) Extremely uneven pollinator pools or perfect floral avoidance of HP represent forms of plant–pollinator specialization and lead to the lack of a CP–HP relationship.
The distribution of shared pollinator visits among co-flowering plants depends on their density and their attractiveness to pollinators (Rathcke, 1983). If plants are equally attractive, pollinators are expected to be recruited only as a function of flower densities in a patch, such that pollinator visitation reflects species’ proportional flower abundance (i.e. neutral interactions). However, when pollinators are a limited resource, differences in relative flower density can disproportionally increase visitation rate to species with high flower densities at the expense of species with lower densities (i.e. competition; Rathcke, 1983; Braun and Lortie, 2019), as pollinators seek to exploit the most abundant resource. On the other hand, rare species (those at low densities) could benefit from increased overall flower abundance if pollinators are most attracted to highly rewarding flower patches, such that pollinator visitation to rare species increases in the presence of other co-flowering species (i.e. facilitation; Ghazoul, 2006). These different types of plant–plant pre-pollination interactions are in turn expected to lead to decreased (e.g. competition) or increased (e.g. facilitation) patterns of CP receipt. However, relative flower abundances cannot only affect CP receipt, but also affect HP delivery (Thomson et al., 2019). For instance, rare species that are facilitated are expected not only to receive more CP but also to receive larger HP loads due to increased pollinator visits to heterospecific flowers, potentially creating a trade-off between conspecific pollen load size and purity (Thomson et al., 2019). Nonetheless, if we only consider relative flower abundance and its effects on visitation rate, then facilitative interactions may be identified by a linear increase in CP (increased pollinator visitation) with increasing HP receipt (increasing visits to heterospecific flowers), as shown by Tur et al. (2016). However, the sign of the relationship (competition versus facilitation) may depend on the accrual rate of HP versus CP (see below).
Pollen transfer, however, depends on more than just visitation rate (reviewed in Minnaar et al., 2018). In particular, pollen transfer can be significantly influenced by inequalities in the efficacies of pollinators visiting flowers and by plant manipulation of pollen acquisition and deposition via floral morphological adaptations (e.g. Herrera, 1987; Conner, 1995; Moreira-Hernandez and Muchhala, 2019). For instance, pollinator pool diversity can be even (all pollinator taxa are equivalently represented) or uneven (some taxa are frequent while others are rare) and when pollinators differ in their effectiveness the degree of evenness can affect pollen receipt (Arceo-Gómez et al., 2016; Koski et al., 2018). Pollinator efficacy, i.e. the capacity of pollinators to transfer pollen from stamens to the conspecific stigma (Freitas, 2013; King et al., 2013), may depend on their behaviour at a flower as well as their constancy during single bouts (Minnaar et al., 2018). On the other hand, floral morphology can mediate the placement of pollen on a pollinator’s body (Herrera, 1987; Huang et al., 2015; Tong and Huang, 2016; Fantinato et al., 2018; Minnaar et al., 2018), and thus the likelihood of pollen deposition on a stigma. For instance, relative positioning of anthers and stigmas can increase CP transfer and reduce the receipt of HP while absence of such floral avoidance strategies is expected to lead to higher HP transfer (Armbruster et al., 2009; Ashman and Arceo-Gómez, 2013; Minnaar et al., 2018; Moreira-Hernández and Muchhala, 2019). Other plant traits, such as pollen morphology, may also affect pollen adherence to, or resistance to grooming from, pollinators (Konzmann et al., 2019) and can be viewed similarly to HP floral avoidance morphology in their influence on pollen transport (Minnaar et al., 2018). In the following paragraphs we discuss specifically how the pollinator pool and floral avoidance can modify the effect of visitation rate and in turn affect the CP–HP relationship and thus influence our interpretation of facilitative versus competitive plant–plant interactions.
We predict that the above processes combine to create different accrual patterns of CP and HP on stigmas resulting in different relationships between CP and HP when flowers are sampled at the end of their lifetimes. So far, however, their influences on CP and HP receipt have only been considered independently from each other (Arceo-Gómez et al., 2016; Tur et al., 2016). As explained above, linear increasing relationships can arise entirely from quantitative differences in the relative availability of pollinators and flowers (Fig. 2A; for examples see Arceo-Gómez et al., 2016; Tur et al., 2016). However, this would only occur if both CP and HP are deposited with each pollinator visit – as pollinator visits increase, the stigma accumulates HP at the same rate as CP. This pattern would be expected when the assemblage of pollinators is even and of similar effectiveness (depositing similar amounts of CP and HP; Arceo-Gómez et al., 2016), but only when the recipient species’ flowers do not display floral adaptations for avoidance of HP. Under this scenario, differences in the effect of co-flowering species presence on the visitation rate to a focal species, i.e. neutral (visits proportional to focal species abundance), facilitative (visits greater than proportional abundance) or competitive (visits less than proportional abundance), are expected to affect the steepness but not the sign of the CP–HP accrual relationship (Fig. 2A). For instance, if the effect of co-flowering species presence is to reduce visitation rate of the focal species, i.e. a competitive interaction, then the slope of the CP–HP relationship is expected to be low (Fig. 2A), as visitors deposit more HP than CP with every visit owing to spending more time on the competitors’ flowers than those of the focal species. In this case, both CP and HP will still increase as more visits are received but HP will accumulate faster than CP (Fig. 2A). In an artificial array of two flower types for instance, Thomson et al. (2019) observed that unattractive flowers receive larger pollen loads but with much higher amounts of HP compared with CP when intermingled with a competitor compared with when they are not. This result indicates that HP can accumulate faster than CP in the presence of competition. Wide among-species variation in the value of CP–HP positive slopes has also been observed within a single community (Tur et al., 2016). Hence, we propose that not only the direction (Tur et al., 2016) but also the strength of the slope of the CP–HP relationship is an important indicator of the potential type of plant–plant interaction taking place (competitive versus facilitative). Furthermore, under extreme asymmetrical competition for pollinators, not only will stigmas of the competitive loser accrue more HP and less CP, but limited space on the pollinators’ bodies may result in a trade-off in transport between CP and HP because higher HP loads will prevent transport of CP. This trade-off can lead to an overall negative relationship between CP and HP receipt (Fig. 2B; for an example see Tur et al., 2016). Thus, we propose that when there is no floral HP avoidance and the effectiveness of the pollinator pool is even, the type of pollinator-mediated plant–plant interactions (competitive versus facilitative) reflected by CP–HP relationships can be differentiated by both the steepness of the positive slope of the CP–HP linear relationship when positive (Fig. 2A) and the negative sign of the slope in the presence of pollen placement trade-offs (Fig. 2A versus 2B). Some observational evidence of these CP–HP relationships exists (Arceo-Gómez et al., 2016; Tur et al., 2016) but these predictions are yet to be confirmed. While we recognize the difficulty of testing these predictions using stigmas collected in the field alone, they could be corroborated with other field data, such as characterizing pollen on pollinator bodies to evaluate pollen transport trade-offs, or they could be tested in experimental settings where floral morphology (e.g. artificial flowers), pollinators and relative flower densities are manipulated (e.g. Thomson et al., 2019). Nonetheless, this set of outcomes illustrates a salient point that a single ecological process, in this case pollinator competition, can yield two different patterns of CP–HP covariation (positive and negative; Fig. 2A, B) and that the same CP–HP pattern (positive) can be reflective of facilitation (Fig. 2A; visit rate greater) or competition (Fig. 2A; visit rate lower) depending on the magnitude of the slope. Thus, interpretation of the functional drivers must be cautious and well informed with respect to floral features and other aspects of patterns of pollen deposition on stigmas.
Quantitative relationships between CP and HP delivery are modified not only by competition and facilitation for visits but also by differences in quality aspects of pollination (amount and purity of CP loads). Specifically, the relationship between CP and HP can be non-linear when the pollinator pool is uneven, i.e. composed of one or few frequent, high-quality pollinators that deliver mostly CP plus several less frequent, low-quality pollinators that deliver CP and HP (or vice versa). Such patterns have been observed in several communities (Arceo-Gómez et al., 2016). Thus, we propose that unequal distributions of CP relative to HP on stigmas will occur as some stigmas receive highly effective pollinators (depositing abundant CP and rare HP) whereas others receive visits by less effective pollinators (carrying small amounts of CP and varying amounts of HP). As most HP would be delivered along with some small amounts of CP during visits by these inefficient pollinators, this scenario leads to an exponentially decreasing relationship of CP with HP receipt (‘uneven’ in Fig. 2C; for an example see Arceo-Gómez et al., 2016). In contrast, if numerous high-quality pollinators deliver small but pure CP loads and infrequent low-quality pollinators deliver large mixed (CP and HP) loads, then CP will show a negative exponential increase with HP (‘uneven’ in Fig. 2D; for an example see Arceo-Gómez et al., 2016). Thus, the quality of pollinators, not just the quantity of visits, affects CP–HP relationships. And specifically, the type of non-linear relationship that occurs with unevenness of pollinators depends on the amount of CP delivered by the most effective pollinators (also see Arceo-Gómez et al., 2016).
Interestingly, when floral avoidance morphology is not perfect at reducing HP deposition by all pollinators in the pool, it differentially impacts their transfer of CP relative to HP, and thus can also lead to non-linear relationships. For instance, when floral avoidance is more effective at reducing HP carried by pollinators bringing small loads than those carrying large loads of pollen a negative exponential CP–HP relationship would result. Large amounts of HP would only be delivered along with large CP loads, even under conditions of pollinator pool evenness and quantitative facilitation (‘present’ in Fig. 2D). Likewise, floral HP avoidance could exacerbate the variance in effectiveness of pollinators in the pool contributing to the non-linearities between CP and HP represented in Fig. 2C, D (e.g. ‘uneven’and ‘present’ in Fig. 2C and ‘even’and ‘present’ in Fig. 2D), among other forms. It is worth noting that the most extreme case of pollinator pool unevenness is equivalent to absence of pollinator sharing (i.e. a single specialist, highly effective pollinator) and for these plants we predict there would be a non-significant CP–HP relationship with low variance in HP among flowers (Fig. 2E; for an example see Arceo-Gómez et al., 2016). A similar pattern would be expected when floral avoidance is perfectly effective and excludes all HP transfer, or when flowers exhibit early deposition of self CP (‘prior selfing’; Lloyd and Schoen, 1992), preventing HP deposition by late-arriving visitors (e.g. Randle et al., 2018). This discussion makes clear that CP–HP relationships could arise from forces unrelated to the degree of competition and facilitation for visits. The described cases are examples of how the CP–HP relationships are strictly influenced by the single or combined action of flower morphology, pollinator diversity and autonomous selfing mechanisms (also see Arceo-Gómez et al., 2016; Minnaar et al., 2018). Thus, future studies should test for the presence of non-linear (in addition to linear) CP–HP relationships to determine whether these factors are at play in pollinator-mediated plant–plant interactions.
In conclusion, while variation in pollinator visitation rates alone can lead to linear CP–HP relationships that could reflect competition or facilitation for pollinator visits (e.g. Tur et al., 2016), we argue against this simplistic perspective on two grounds. First, CP–HP relationships are influenced by numerous factors other than the nature of the pre-pollination interaction – of which we have focused on two (pollinator pool and floral morphology) – that can result in complex (and non-linear) relationships (Fig. 2C–E). Second, both positive and negative slopes could be outcomes of competitive interactions, and positive slopes could arise from neutral, facilitative or competitive interactions (Fig. 2A, B). Thus, additional experiments are needed to avoid misinterpretation of the role of pollinator-mediated facilitative and competitive interactions in nature. We argue that to tease apart these pollinator-mediated plant–plant interactions, one will require not only knowledge of CP–HP patterns but of aspects of the reproductive biology (e.g. floral HP avoidance strategies, potential for autogamous self-pollen deposition, level of pollinator specialization) of the species being studied as well as manipulations to verify the predicted interpretations. For instance, when a positive CP–HP relationship is observed (Fig. 2A), manipulations of the whole floral community and/or key interacting species (i.e. those identified as sharing pollinators from the identity of pollen of the stigma; see below) can be used to test whether the pre-pollination interactions are competitive or not. If removal of heterospecifics leads to a higher CP–HP slope by the focal species, then pre-pollination interactions were competitive. However, if removal leads to a lower slope of the CP–HP relationship for the focal species, then pre-pollination interactions were facilitative. These community-level experiments can be logistically daunting, but by identifying key facilitators/competitors’, effects can be simultaneously evaluated on multiple focal species. Focused manipulative experiments will also avoid the time-consuming task of observing pollinator visits on a wide range of species sometimes leading to insufficient sampling. Likewise, manipulations of the pollinator pool evenness can be used to confirm its role in creating non-linear relationships (Fig. 2C, D). For instance, selective removals of pollinators (e.g. Brosi and Briggs, 2013) could be used to increase evenness in the pollinator pool, an effect predicted to reduce non-linearities under competitive pre-pollination interactions (Fig. 2C), potentially revealing a negative relationship resulting from CP–HP trade-offs (Fig. 2B). Finally, manipulation of focal species floral traits, for instance style length (e.g. Creswell, 2000), can be used to test whether floral avoidance contributes to non-linearities in CP–HP relationships (Fig. 2C, D). If changing the height of the stigma alters the CP–HP relationship, then manipulations of flower abundance (see above) can be used to determine the nature of pre-pollination interactions (i.e. via visitation). Pairing CP–HP patterns with a judicious combination of experimental approaches can provide more rigorous information about the nature of plant–plant interactions than either alone.
PROCESSES THAT UNDERLIE CP TUBES/GRAIN–HP RELATIONSHIPS
A second, often overlooked, relationship that can be observed from flowers at the end of their lifetime is that between CP tubes in the style and the load of CP and HP on the stigma (e.g. Briggs et al., 2016). The relationship between the number of CP tubes and the number of CP grains alone can provide information on the CP quality aspect of pollination (Kalla and Ashman, 2002; Parra-Tabla and Bullock, 2005; Alonso et al., 2012). The relationship between the proportion of CP grains that produce a tube (i.e. CP tubes/grain) and HP (Fig. 1B) is particularly relevant because it informs on the consequences of HP receipt for effective fertilization and is thus central for assessing the final outcome of pollinator-mediated competitive or facilitative interactions. Thus, consideration of both the quality of CP delivered to the stigma and the potential CP–HP interactions that occur after deposition is essential for understanding the contribution of post-pollination interactions to plant evolution and community structure (Morales and Traveset, 2008; Ashman and Arceo-Gómez, 2013; Briggs et al., 2016). Below we briefly describe the factors that can influence CP–HP interactions on the stigma and thus our interpretation of the HP–CP tubes/grain patterns observed.
The quality of CP that is delivered to a stigma is affected by genetic and environmental factors. Environmental conditions during pollen development and dehiscence, such as temperature, drought and UV irradiance, can reduce pollen grain viability and tube vigour (e.g. Kakani et al., 2002; Koski and Ashman, 2015). Pollen genotype can directly determine whether CP grows a tube on a given stigma, e.g. genetic self-incompatibility (Rea and Nasrallah, 2008, Vaughton et al., 2010), and can interact with the environment to determine CP potency (e.g. Kakani et al., 2002). The genetic relatedness (e.g. outcross versus self) of CP on a recipient stigma can be dictated by plant floral traits (herkogamy or dichogamy), population genetic structure/density, or the behaviour of pollinators within or among plants (e.g. Mazer et al., 2010). Even changes in pollinator behaviour in response to the presence of heterospecific plants can alter the genetic composition of CP delivered to stigmas (e.g. increased selfing in the presence of competitors; Flanagan et al., 2009). Because outcross pollen can be more vigorous, and produces tubes at higher rates than self pollen, the genetic relatedness of CP alone can affect the relationship between the number of CP tubes and grains (e.g. Parra-Tabla and Bullock, 2005, Arceo-Gómez and Ashman, 2014a).
Interactions between HP and CP grains on the stigma, or tubes within the recipient style, can also influence the quality of pollination. Heterospecific pollen can reduce CP tubes as a result of allelochemical or physical interference (Morales and Traveset, 2008; Ashman and Arceo-Gómez, 2013; Wipf et al., 2016; Takemori et al., 2019). Heterospecific pollen can sometimes lead to increased seed production (Arceo-Gómez et al., 2019; Suárez-Mariño et al., 2019), possibly reflecting increased CP tubes/grain as a result of the ‘herd’ or ‘mentor’-like effect (as in CP; Cruzan, 1990; Niesenbaum, 1999). Seed production may also increase via release of biochemical compounds that influence pollen tube growth (i.e. spermidine; Palmer‐Young et al., 2019) when HP germinates or bursts on the stigma (e.g. Prieu et al., 2016; Wipf et al., 2016). In multispecies loads of HP, antagonistic effects between HP grains can lead to non-additive effects on CP performance (Arceo-Gómez and Ashman, 2011). And finally, because HP can interact more strongly with self CP than outcross CP (Arceo-Gómez and Ashman, 2014a), the quality of CP deposited with HP can affect the relationship between CP tubes/grain and HP.
Once again, pollen–pollen interactions on the stigma could combine to create different relationships between CP tubes/grain and HP under natural pollination conditions, but their actions have often been evaluated independently from each other. For instance, a decline in CP tubes/grain with increasing HP could result from HP interfering with CP germination or tube growth (‘negative’ in Fig. 3A), whereas an increase could occur if HP has positive effects on CP germination or growth (‘positive’ in Fig. 3B; see Tur et al., 2016 for examples). However, these same types of relationship could also arise when there is no effect of HP directly on CP but rather the presence of heterospecifics in the community changes the quality of CP deposited by pollinators. For instance, when increased presence of heterospecific plants leads to increased selfing and thus lower-quality CP deposited with HP (‘decrease’ in Fig. 3A), or alternatively when pollinators fly longer distances between rare species and more outcrossed pollen is brought along with increased deposition of HP (‘increase’ in Fig. 3B). Opposing impacts of CP quality and CP–HP interactions (e.g. increase in CP quality along with an increase in HP interference) can create the lack of a significant relationship between CP tubes/grain and HP (‘increase’ and ‘negative’ in Fig. 3C; see Tur et al., 2016 for examples), so it is important not to disregard such relations entirely by assuming neutral effects (‘no change’ and ‘none’ in Fig. 3C) when a lack of significant slope is observed. Interestingly, when there are dose-dependent effects of HP on CP tube growth (e.g. allelopathic effects; Thomson et al., 1981; Arceo-Gómez et al., 2018b) or when herd effects only occur after surpassing a threshold pollen load size, non-linear responses to HP are possible (Fig. 3D–F, respectively). If HP only interferes with CP germination after a relatively large load size is reached, then non-linear declining threshold effects could result (Fig. 3D). But if HP interferes with CP intensely even in small amounts then pronounced declines in CP tubes/grain with increasing HP could result (Fig. 3E; for an example see Thomson et al., 1981). If HP has positive effects, then the opposite trend would be expected (Fig. 3F). Explicating these possibilities makes clear that the CP tubes/grain versus HP relationship can be complex. Teasing apart the different factors that drive these relationships will require careful experimentation where the origin, identity and quality of pollen deposited on stigmas is manipulated (for examples see Arceo-Gómez and Ashman, 2011, 2014a ). Nevertheless, data on the relationship between CP tubes/grain and HP provide crucial insight into the consequences of pollinator sharing that occur after pollen deposition (e.g. effects of CP quality and HP interference, and mentor effects). Thus, CP tubes/grain remains an important proxy that deserves more empirical attention and careful interpretation.
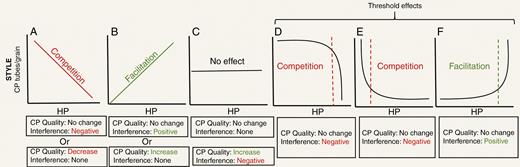
Patterns of conspecific (CP) tubes/grain versus heterospecific pollen (HP) received on stigmas of spent flowers and potential drivers of variation in these patterns. Potential drivers are CP quality and HP interference with CP, with positive interaction noted in green and negative ones in red for emphasis. In (A) increasing interference or decreasing quality of CP can lead to a negative linear CP tubes/grain–HP relationship, which can be interpreted as forms of competitive interaction. In (B) positive linear interaction between CP–HP or increasing quality of CP can lead to a positive CP tubes/grain–HP relationship, which can be interpreted as a facilitative interaction. In (D) and (E) threshold effects of HP load size on the stigma are driving non-linear patterns and no CP quality effects are at play. Dotted lines reflect the threshold number of HP grains required to cause a competitive (D, E, inference only at large or small HP load sizes, respectively) or facilitative (F, mentoring at high HP) effects.
ASSUMPTIONS AND ADDITIONAL CONSIDERATIONS
The insights gained from pollen composition on stigmas depend on how closely the underlying assumptions are met. Below we consider how several assumptions may affect the dynamics and interpretation of pollination processes.
Adequacy of sampling effort
The first assumption is that large sample sizes afforded by use of stigmatic pollen loads are adequate to fully characterize the multipartite relationships of interest. Lack of power, however, could lead to incorrect interpretations of the dominant pre-pollination processes, such as concluding that a linear relationship exists (Fig. 2A) when it does not, or concluding that a relationship does not exist (‘no change’ and ‘none’ in Fig. 3C) when a non-linear one does (Fig. 3D–F). The large flower-to-flower intraspecific variance in stigmatic pollen loads that is often observed (Alonso et al., 2013; Arceo-Gómez et al., 2016; Thomson et al., 2019) suggests that appropriate sample sizes need to be seriously considered, although this has yet to become commonplace for stigmatic load data sets. However, two studies provide some insight into adequate sampling for these purposes. Alonso et al. (2012) recommends 150 stigmas per species to assess non-linearities in CP tubes/grain relationships, whereas Arceo-Gómez et al. (2018a) recommend sampling a minimum of 50 stigmas per species to capture HP diversity. These sample size targets can be used as starting points for future studies, although rarefaction approaches will also be highly effective for assessing the sampling adequacy of a given study (e.g. Arceo-Gómez et al., 2018a). Evaluating power will be a key component of any study using these proxies.
Influences of temporal and spatial variation
Another implicit assumption in the analysis of stigmatic pollen loads from spent flowers is that CP and HP arrive somewhat simultaneously, or at least have the same possibility of arriving throughout flower life, as might be the case for pollinators with diffuse pollen placement (Minnaar et al., 2018). The arrival time of HP and CP cannot, however, be inferred from collections made at flower senescence, but this timing could affect pollen load composition as well as pollen tube growth. For instance, flowers with pronounced autonomous autogamy (prior or delayed self-pollination relative to outcross pollination; Dole, 1990; Lloyd and Schoen, 1992; Randle et al., 2018) could have stigma loads that are not purely pollinator-mediated, and thus differ dramatically in the time of self or outcross pollen arrival even in the absence of pollinator visits. Self and outcross CP grains can differ in their tube growth rates (e.g. Sorin et al., 2016) so different schedules of their delivery will affect interpretations of quality aspects of pollination and CP tubes/grain–HP relationships. Timing of HP delivery can affect the impact of HP on CP tubes/grain: simultaneous HP deposition has a greater negative effect on CP than staggered deposition (Bruckman and Campbell, 2014). Finally, autogamy will reduce flower-to-flower variance in CP receipt, and thus could obscure patterns of variation. For example, prior selfing could lead to high CP with low HP, as in Fig. 2E. As a consequence, it will be important to take the mode of delivery of self CP into consideration when evaluating CP–HP covariance as well as for the interpretation of the CP tubes/grain–HP relationship.
Finally, it is also implicitly assumed that the multispecies communities under study lack pronounced spatial structuring of the interacting plants and/or that pollinators disregard such structure if it exists. The evidence for impact of these on pollen transfer, however, is mixed. Strong spatial structure in simple communities (e.g. illustrated with arrays of artificial flowers; Thomson et al., 2019) can impact HP and CP receipt, but local community factors did not affect HP receipt in three wild communities (Table 3, ‘conspecific density effect’ in Arceo-Gómez et al., 2016). The degree to which spatial structure affects the proxies described herein can be tested with observational data of local plant distributions (e.g. Arceo-Gómez et al., 2016) or via manipulations (e.g.Thomson et al., 2019).
Influences of pollen identity and diversity
The proxies as described thus far implicitly assume that HP behaves as a uniform population of interspecific pollen. The specific identities and phylogenetic relationships of the HP donors (to each other and to the recipient), however, can result in varied individual and non-additive combinatorial effects on recipient CP tube growth and seed production (Arceo-Gómez and Ashman, 2011; Arceo-Gómez et al., 2016, 2019). Moreover, if HP diversity increases with load size, as seen in Arceo-Gómez et al. (2016), and HP diversity affects CP tubes/grain (Arceo-Gómez and Ashman, 2011), then non-linear relationships between them may be more common in nature than not (e.g. Figs 2D, E and 3D–F). Thus, a full understanding of pollination interactions from the community perspective will require characterization of the diversity and composition of HP in addition to abundance on the stigma, as well as testing whether the composition of the HP stigmatic community affects CP tubes/grain versus HP relationships (e.g. Arceo-Gómez and Ashman, 2011).
CONCLUSIONS
Even in the light of the complex relationships described here, stigmatic pollen loads can add important information to our understanding of pollination processes at the community scale and in the wild. They directly address post-pollination interactions that cannot be captured by visitation metrics alone. For instance, facilitation for pollinator visits that does not lead to higher pollen deposition represents only ‘apparent’ facilitation because it cannot lead to higher reproduction. Here CP and HP on stigmas would be more reliable than visits as an indicator of the net interaction. In addition, evaluating the CP tubes/grain and HP relationship provides relevant information on whether a positive relationship between HP and CP receipt ultimately leads to a negative fitness effect or not. Thus, stigmatic proxies are valuable for understanding pollinator-mediated plant–plant interactions in multispecies communities, but we urge researchers to be clear about what can and cannot be inferred from these data alone. In particular, we highlight that competition and facilitation can lead to the same CP–HP outcomes. To this end, we suggest that validation of hypothesized mechanisms (with knowledge of the natural history and reproductive biology of species and via experimentation) are required for a true understanding of the ecological process underlying the patterns observed on stigmas. We also argue, however, that stigmatic pollen loads can provide insight into contemporary pollinator-mediated plant–plant interactions, as well as those that occurred in the past. For instance, Johnson et al. (2019) used pollen on stigmas sampled from herbarium sheets to infer historical pollination interactions. Thus, stigmatic pollen loads represent a crucial tool for pollination ecologists faced with describing community-wide pollination interactions that are rapidly being transformed globally by human activities (IPBES, 2016).
ACKNOWLEDGEMENTS
The authors thank P. Sosenski for discussions, and H. Briggs, three anonymous reviewers and T. Schwarzacher for comments that improved the manuscript. Logistical support was provided by National Science Foundation USA-DEB1452386 and Univeristy of Pittsburgh Dietrich School of Arts and Sciences to T.L.A., East Tennessee State University Research Development Committee grant to G.A.G. and Consejo Nacional de Ciencia y Tecnología grant 248406 to V.P.T.



