-
PDF
- Split View
-
Views
-
Cite
Cite
André Tavares Corrêa Dias, Bruno H P Rosado, Francesco De Bello, Nuria Pistón, Eduardo A De Mattos, Alternative plant designs: consequences for community assembly and ecosystem functioning, Annals of Botany, Volume 125, Issue 3, 14 February 2020, Pages 391–398, https://doi.org/10.1093/aob/mcz180
Close - Share Icon Share
Abstract
Alternative organism designs (i.e. the existence of distinct combinations of traits leading to the same function or performance) are a widespread phenomenon in nature and are considered an important mechanism driving the evolution and maintenance of species trait diversity. However, alternative designs are rarely considered when investigating assembly rules and species effects on ecosystem functioning, assuming that single trait trade-offs linearly affect species fitness and niche differentiation.
Here, we first review the concept of alternative designs, and the empirical evidence in plants indicating the importance of the complex effects of multiple traits on fitness. We then discuss how the potential decoupling of single traits from performance and function of species can compromise our ability to detect the mechanisms responsible for species coexistence and the effects of species on ecosystems. Placing traits in the continuum of organism integration level (i.e. traits hierarchically structured ranging from organ-level traits to whole-organism traits) can help in choosing traits more directly related to performance and function.
We conclude that alternative designs have important implications for the resulting trait patterning expected from different assembly processes. For instance, when only single trade-offs are considered, environmental filtering is expected to result in decreased functional diversity. Alternatively, it may result in increased functional diversity as an outcome of alternative strategies providing different solutions to local conditions and thus supporting coexistence. Additionally, alternative designs can result in higher stability of ecosystem functioning as species filtering due to environmental changes would not result in directional changes in (effect) trait values. Assessing the combined effects of multiple plant traits and their implications for plant functioning and functions will improve our mechanistic inferences about the functional significance of community trait patterning.
Introduction
Trait-based ecology has gained increasing attention during the last decades. By focusing on functional traits, i.e. any morphological, physiological or phenological feature measurable at the individual level affecting fitness and/or influencing ecosystem processes or other organisms (Hortal et al., 2015; Carmona et al., 2016; Rosado et al., 2016), one should be able to provide generalized predictions on community structure and its effect on ecosystem functioning. Community trait patterning is widely used to infer mechanisms of community assembly, where detection of trait underdispersion, overdispersion and shifts in community-level trait average values are interpreted as consequences of processes such as environmental filtering, competition and disturbance (Cavender-Bares et al., 2009; Götzenberger et al., 2012; HilleRisLambers et al., 2012). Existing frameworks often do not account for the fact that an individual’s fitness results from a combination of traits, including multiple trade-offs (Marks, 2007; Violle et al., 2007; Rosado and de Mattos, 2010; Laughlin and Messier, 2015). Traits are usually examined individually or combined into metrics that summarize combined trait dimensions (Wright et al., 2004; Reich, 2014). However, multidimensional phenotypes, including trait covariance and interactions between traits, are likely to play a key role in community assembly because biotic and environmental filters act on multiple traits simultaneously (Laughlin et al., 2015; Dwyer and Laughlin, 2017; Piston et al., 2019).
An important and overlooked consequence of multidimensional phenotypes is that phenotypically dissimilar species can have a similar performance in the same environment (Marks, 2007). Such alternative organism designs (Marks and Lechowicz, 2006), i.e. the existence of distinct combinations of traits leading to the same function or performance in a given environment, are considered as an important mechanism driving the evolution and maintenance of the large quantitative variation of traits observed in plants and animals (Marks, 2007; Wainwright, 2007; Shoval et al., 2013). Similarly, alternative designs can potentially be an important mechanism of community dynamics. Different suits of traits can lead to a similar response to environmental factors or similar competitive abilities. Taking this into account substantially changes our expectations of trait patterning as a consequence of community assembly processes (Marks, 2007). However, although alternative designs are commonly observed in nature, they have not yet been considered consistently in the scope of the theory of ecological communities.
Here, we discuss how overlooking the potential effect of alternative designs can compromise our ability to detect the mechanisms responsible for species coexistence and to understand the effects of species on ecosystems. Additionally, we use the concept of trait integration level to hypothesize which types of traits should be more or less directly coupled with species performance. Finally, we suggest analytical and conceptual approaches that can help in incorporating alternative designs into community and ecosystem ecology.
ALTERNATIVE DESIGNS AND TRAIT INTEGRATION LEVEL
Alternative designs can be understood as different solutions for the same problem (i.e. coping with an ecological restriction or performing a function). For instance, the frequent occurrence of both annuals and succulent plant species in highly seasonal ecosystems indicates contrasting strategies which allow drought avoidance and tolerance, respectively (Lambers et al., 2008). In sites subjected to high grazing, both prostrate hemicryptophytes and spiny phanerophytes can be found (van der Meijden, 2005). Different strategies can also result in similar reproductive success. In the context of seed dispersal, different structures can favour dispersal by the same agent. Achenes are dispersed by wind either due to the presence of the flat expansions (i.e. wings) in the samaras of Acer species or due to modified sepals forming plumed structures (i.e. the pappus) in Asteraceae species (van der Meijden, 2005). Similarly, colourful berries, dehiscent fruits with bright red pulp and even mimetic seeds can attract birds that disperse the seeds. Different strategies can also favour competitiveness. When competing for light, both fast-growing tall plants and short shade-tolerant species can thrive in the community (Falster and Westoby, 2005). Beyond these few examples, alternative organism designs are no doubt a widespread phenomenon in plants, as evidenced by the high quantitative variation of trait values among species within a given site (Moles et al., 2007; de Bello et al., 2009).
The importance of alternative designs has gained much attention in the scope of evolutionary biology (Marks and Lechowicz, 2006; Marks, 2007; Wainwright, 2007). Traditional views postulate that the environment should select individuals with traits allowing them to cope with the prevailing conditions of a site. Accordingly, natural selection is traditionally modelled using single trade-off models for seeking patterns of fitness optimization (Parker and Smith, 1990; Adler et al., 2014). This leads to the idea of only one optimal combination of trait values for a given environment. As whole-organism functioning is not the result of any single trait but of an array of traits, including multiple trade-offs among them (i.e. multidimensional phenotypes; Meziane and Shipley, 2001; Marks and Lechowicz, 2006), there will often be multiple possible solutions to the same evolutionary challenge (Lewontin 1978). If there are multiple fitness optima in a given environment, the particular array of traits displayed by a lineage, and the evolutionary path taken by the lineage to reach this local optimum, will depend on phylogenetic constraints and chance (Marks, 2007). The existence of such alternative functional designs can lead to flexibility in trait selection and evolution, as many combinations of trait values are possible for the same fitness under a given environmental condition (Wainwright, 2007). In this way, alternative designs comprise an important mechanism behind the evolution and maintenance of trait diversity (Marks, 2007; Wainwright, 2007).
The same reasoning applies when studying environmental selection on an ecological time scale. Alternative strategies, comprising widely different arrays of traits, can lead to the same response to the environment. Consequently, single traits and function might not have a direct relationship (Wainwright, 2007; Laughlin and Messier, 2015), allowing very dissimilar species to be found side by side (Cavender-Bares et al., 2004; Wainwright, 2007; Bermúdez and Retuerto, 2013; Silva et al., 2018). In this way, two species can be considered very different when looking at single traits but they might actually display very similar resistance to a given environmental condition or competitive ability to a given resource (Rosado and de Mattos, 2017). In an ecological context, species differences in vital rates, i.e. survival, growth or reproduction (Violle et al., 2007; Adler et al., 2014), can ultimately lead to differences in relative abundances, which are often taken as a surrogate for ecological performance (performance hereafter) when studying species coexistence (see Fig. 1; Shipley et al., 2016). Similar performance can be the result of contrasting strategies that enhance different components of performance, with a well-established trade-off between them (e.g. trade-off between survival and reproduction). Nevertheless, very dissimilar species can show a similar magnitude on the same performance component. For instance, there are many mechanisms (and trait arrangements) by which plants can survive drought (Pivovaroff et al., 2016). Therefore, alternative designs can act both among and within performance components. The open challenge lies in the identification of different trait combinations that cause species to have a similar performance in a given ecological condition.
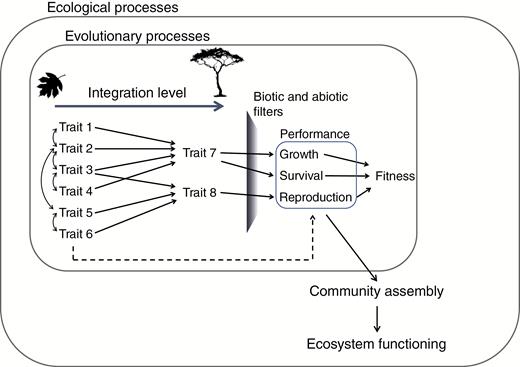
Conceptual model modified from Arnold (1983) to include the concept of the organism integration level (Marks, 2007), ranging from organ-level traits (traits 1–6) to whole-organism traits (traits 7 and 8). Whole-organism traits result from different arrays of organ-level traits, including their multiple trade-offs (double-headed curved arrows). Solid arrows represent the causal relationships between phenotypical traits and performance and their consequences for fitness (evolutionary processes) and community assembly (ecological processes). The relationship between organ-level traits and performance (and consequently fitness and community assembly) is considered indirect (dashed line), as whole-organism traits more properly represent the integrated functioning of the organism. Therefore, filters should act directly on whole-organism traits and only indirectly on organ-level traits. Additionally, the community assembly process (and the resulting trait composition) can strongly influence ecosystem functioning as illustrated by the response and effect framework proposed by Suding et al. (2008).
Fundamental to tackling this challenge, and to incorporating alternative designs in the analyses of community assembly and ecosystem functions, is the concept of trait integration level (Marks, 2007). Essentially, traits are expected to be hierarchically structured, ranging from organ-level traits to whole-organism traits (Fig. 1). Whole-organism traits reflect the integrated functioning of the organism (e.g. growth rate and desiccation resistance), and are hypothesized to show a direct relationship with species response to environmental factors and, therefore, performance under different environmental conditions (Marks, 2007). In turn, whole-organism traits often depend on the combined effect of multiple underlying organ traits, e.g. different trait combinations determine drought tolerance, which is itself a trait resulting from an integration of (potentially) multiple traits. Therefore, traits measured on higher levels of organism integration (i.e. whole-organism traits) should be more susceptible to environmental selection, while organ-level traits are expected to be more strongly evolutionarily conserved (Marks, 2007). Accordingly, whole-organism traits are better predictors of performance in comparison with organ-level traits (Yang et al., 2018). The use of the concept of the trait integration level requires the identification of the combinations of organ-level traits that provide the integrative functional strategy of a species. Below, we explore how alternative designs combined with the concept of trait integration level can be used to further advance our mechanistic understanding of community assembly and ecosystem functioning.
CONSEQUENCES FOR COMMUNITY ASSEMBLY
Much work on community assembly theory has emphasized community trait patterning as the result of assembly processes, where underdispersion and overdispersion of trait values, as well as shifts in the average trait value in a community, are interpreted as the result of assembly processes (Götzenberger et al., 2012; HilleRisLambers et al., 2012). Functional diversity (FD) indices based on one or multiple traits are often used to test such patterns. Underdispersion, when co-occurring species are ecologically similar, is characterized by FD values lower than expected by chance. Conversely, overdispersion, when co-occurring species are ecologically different, is characterized by FD values higher than expected by chance. A strong relationship between traits and performance is a fundamental assumption for inferring assembly processes from trait patterning (Shipley et al., 2016). However, such tests often do not account efficiently for interactions between traits and are, therefore, not an ideal tool to describe the relationship between multidimensional phenotypes and performance. Here we use the concept of dynamic adaptive landscape proposed by Laughlin and Messier (2015) as a general framework to identify the consequences of different types of relationships between traits and performance for trait patterning as a result of distinct community assembly processes. In addition, we use the concept of trait integration level to generate hypotheses on which kind of trait should be important for different assembly processes.
Environmental Filtering
Consider first the adaptive landscape in a particular habitat. Figure 2 presents different relationships between trait arrays and performance. For the sake of simplicity, we illustrate the landscape determined by two traits, but the same rationale could be applied to several traits. Most commonly, researchers assume simple relationships between traits and performance, either when a single trait is directly related to performance (Fig. 2A) or when one specific combination of traits results in higher performance (Fig. 2B). This latter is often observed when the traits related to performance are highly correlated, describing trait syndromes or ecological strategies. This leads authors to condense multiple traits on axes of multivariate analyses synthesizing correlated traits (Westoby et al., 2002), instead of assessing the potential interactions between these and other traits in determining performance. For example, specific leaf area (SLA) and leaf nitrogen content are often correlated, and both contribute to similar ecological functions. The two traits are frequently combined into one axis of leaf trait variation (Wright et al., 2004). Yet, approaches with strong emphasis on reducing the dimensionality of trait space neglect the possibility of more complex relationships between traits and performance (Laughlin and Messier, 2015).
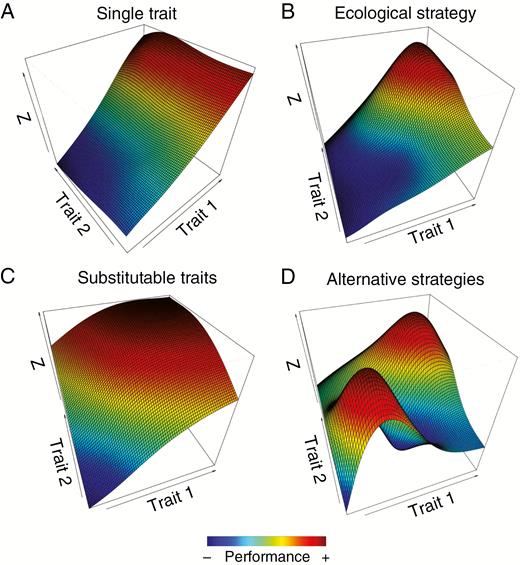
Adaptive landscapes showing fitness values (colour scale) in the space defined by two traits. The figure displays both simple and complex relationships between traits and fitness. Straightforward relationship between trait(s) and fitness are displayed when one single trait (A) or one single combination of trait values (ecological strategy) determines fitness (B). Complex relationships between traits and fitness are displayed when different combinations of trait values show similar fitness (i.e. alternative designs). This happens when traits are substitutable in conferring a function or response to environmental factors (C) or when different ecological strategies provide similar fitness or function (D).
Other scenarios include traits that can be substitutable in conferring a function or response to environmental factors (Fig. 2C). Substitutable traits are traits that jointly contribute to performance in a way that an increase in one trait can compensate for a decrease in the other, and vice versa. In such cases, high values of one trait or the other will lead to high performance values. In this way, many combinations of these traits can lead to equivalent performance resulting in a large adaptive plateau. For example, Valladares et al. (2002) showed that constraints on light capture due to phyllotactic patterns can be offset by crown structural traits, such as internode length and branching angles. In this way, species with contrasting phyllotaxy can show similar light harvesting efficiency. Additionally, two or more adaptive peaks in the landscape, leading to alternative strategies, indicate different combinations of traits with similar performance (Fig. 2D). Recently, Pivovaroff et al. (2016) showed that co-occurring species display distinct strategies to survive drought, which are related to different combinations of a wide array of traits (i.e. deciduousness, root depth, sap-wood capacitance, photosynthetic stems and vulnerability to xylem cavitation). Both substitutable traits and alternative strategies can be considered as alternative organism designs.
Environmental filtering is an important process shaping community structure (Vellend, 2010; HilleRisLambers et al., 2012). If species able to survive at a site share similar traits conferring them with resistance to the abiotic conditions, this should result in trait underdispersion in the community. Clearly, considering the concept of alternative designs, species could be different in terms of single traits, while being almost functionally equivalent due to distinct combinations of traits. Thus, by looking at single traits only, the role of environmental filtering might be not fully appreciated.
By examining the relationships between multidimensional phenotypes and performance in changing (or between contrasting) environmental conditions, it is possible to show that the trait patterns resulting from environmental filtering depend entirely on the type of adaptive landscape. Changing environmental conditions could simply shift the position of the performance peak in the same adaptive landscape. For instance, along an environmental gradient, the performance peak in Fig. 2B could shift from high values of both traits 1 and 2 to low values of traits 1 and 2. Alternatively, a trait that was not important in determining performance could become so in a different environment, resulting in changing the adaptive landscape from that in Fig. 2A to that in Fig. 2B. These changes in the adaptive landscape would lead to directional shifts in community-weighted mean trait (CWM) values and decreased FD values, which is in accordance with the usual interpretation of such community trait patterning as indicative of environmental filtering (Cornwell and Ackerly, 2009; Kraft et al., 2015a).
However, changing environmental conditions could also change the type of adaptive landscape from one trait directly related to performance (Fig. 2A) to a more complex landscape, with the appearance of alternative designs showing two or more performance peaks (Fig. 2D). Such changes to more complex adaptive landscapes can have important consequences for trait patterning reflecting community assembly and can be detected by a number of developing techniques (e.g. Laughlin et al., 2015; Carmona et al., 2016; Pistón et al., 2019). Recently, Silva et al. (2018) showed that moss communities on rocky outcrops did not show reduction of FD or directional changes in CWM values along increasing drought gradients. They showed that the presence of different combinations of traits either increasing water uptake (e.g. concave leaves and hyalocist cells) or reducing water loss (e.g. imbricate leaves and revolute margin of leaves) lead to this non-response of FD to drought. In theory, alternative designs could even lead to an increase, rather than a decrease, in FD of communities experiencing environmental filtering. This is in agreement with the high quantitative variation in traits of species co-occurring in harsh environmental conditions (Rosado and de Mattos, 2010; Freschet et al., 2011; Bermúdez and Retuerto, 2013, 2014; Pivovaroff et al., 2016). However, although the role of alternative design as a driving force increasing FD is well recognized in the evolutionary scope (Marks, 2007; Wainwright, 2007), it is rarely addressed under the scope of community assembly theory (Rosado and de Mattos, 2010, 2017). In fact, when alternative strategies or substitutable traits are observed in harsh environments, mechanisms based on competition (limiting similarity) are often used to explain high values of FD (Stubbs and Wilson, 2004; Bermúdez and Retuerto, 2013, 2014).
The concept of organism integration level could explain this potential duality of environmental filtering resulting in both underdispersion and overdispersion of phenotypes. We should expect phenotypic underdispersion only when evaluating traits directly related to performance under environmental selection. Therefore, whole-organism traits, reflecting the integrated organism response to a given environmental factor, are more likely to converge in a community subjected to environmental filtering (Marks, 2007). This expectation should not necessarily hold for organ-level traits, as substitutable traits and alternative strategies can play an important role in how species respond to environmental conditions. This was exemplified by Rosado and de Mattos (2010), who showed that community dominance in a Brazilian sandy vegetation is determined by the capacity for maintaining high values of plant water potential. On the other hand, dominant species strongly diverge in leaf traits such as SLA (Fig. 3). Considering the hierarchical organization of traits, we should expect phenotypic underdispersion of organ-level traits in special cases when the organ-level trait reflects an integrated functioning of the organisms and there is potentially only one strategy that enables species to survive under the specified environmental conditions. This implies that when using community trait patterning to investigate environmental filtering, researchers should look at whole-organism traits that are directly related to the organism’s response to the environmental factor of interest (e.g. water and nutrient use efficiency, growth rate, etc.). Determining which whole-organism traits are important in different ecological conditions might be challenging in practice, but assessing species response to changing environmental conditions using performance traits, such as tolerance to drought (Moretti et al. 2017), can provide a first estimation.
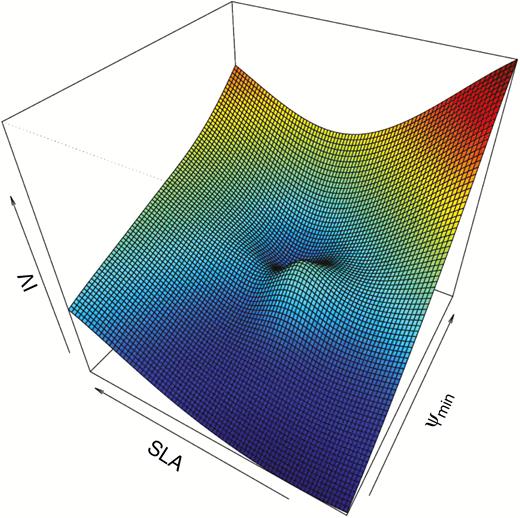
Adaptive landscape showing the phytosociological importance value (IV) as a performance proxy. Dominant species converge in minimum plant water potential (Ψ min, MPa, whole-plant trait), but strongly diverge in specific leaf area (SLA, cm2 g–1, single-organ trait). The adaptive landscape is based on trait measurements of ten woody species in the Brazilian sandy coastal plains (Rosado and de Mattos, 2010, 2018).
Species Coexistence
Biotic interactions are also a fundamental driver of community assembly, determining which species are able to establish and persist in the community (Mayfield and Levine, 2010). In contemporary species coexistence theory (Chesson, 2000), both the relative fitness differences of species, leading to competitive hierarchies, and stabilizing niche differences, leading to resource partitioning, are considered important mechanisms determining species coexistence. Thus, a key task is to understand to what extent such mechanisms depend on functional traits (HilleRisLambers et al., 2012) and on alternative designs. The first existing studies (Pistón et al., 2019) show that alternative designs can be important to define fitness differences and similarities between species. In this context, adaptive landscapes can be understood as a consequence of the interaction milieu (sensuMcGill et al., 2006), indicating which combinations of trait values provide a competitive advantage or minimize niche overlap in a given community (Chesson, 2000). This means that the adaptive landscape depends on the community composition (neighbouring potential competitors), but also on the environmental conditions as it can change the relative fitness differences of species, i.e. ‘differences between species that predict the outcome of competition’ (HilleRisLambers et al., 2012).
According to the theory of limiting similarity (MacArthur and Levins, 1967), coexisting species should diverge in the traits related to the use of limiting resources resulting in high stabilizing niche differences, leading to higher intraspecific competition than interspecific competition (HilleRisLambers et al., 2012). In this case, a similar performance is promoted by distinct resource use strategies, which can result in resource partitioning in space or time. For example, according to the storage effect mechanism, species can persist in a site by either growing quickly and profiting from pulses of favourable conditions, but growing rather intermittently, or, alternatively, growing more slowly but steadily, showing a conservative resource use syndrome. These different resource use strategies may support local coexistence by increasing stabilizing niche differences (Angert et al., 2009; Májekova et al., 2014). Similarly, alternative designs might cause trait divergence, allowing plants to achieve local adaptation by different resource use strategies, thus supporting both coexistence and adaptation to local conditions. Whether the trait divergence was primarily caused by competition or by alternative designs, it would also result in the adaptive landscape of Fig. 2D, where high performance is the result of a distinct combination of traits. This is in agreement with the usual interpretation of higher FD than expected by chance as a sign of niche differentiation. Importantly, traits should be related to resource use strategy, which is performed by different plant organs, such as root traits for nutrient and water uptake, and leaf traits for light harvesting and processing. In this way, traits indicating limiting similarity are, in many cases, organ-level traits and, therefore, are not expected to separately show a strong direct relationship with performance. In such cases, to unravel limiting similarity, it might be necessary to consider multidimensional phenotypes and include possible multimodal relationships between traits and performance (Laughlin et al., 2015). The competition experiment performed by (Kraft et al., 2015b) illustrates these ideas. They showed that traits, when considered separately, are poorly correlated with stabilizing niche differences, and only by considering combinations of traits were they able to adequately describe species niche differences that promote coexistence. Contrastingly, some, often whole-plant, traits such as phenology, canopy shape and plant height are well correlated with average fitness differences, indicating competitive dominance. Additionally, and in particular in combination with other traits, some whole-plant traits might also have an important role in resource partitioning, e.g. plant height and architecture affect space and light partitioning and phenology influences resource partitioning in time (Kraft et al., 2015b).
CONSEQUENCES FOR ECOSYSTEM FUNCTIONING
Incorporating the alternative design concept in the response and effect framework (Suding et al., 2008) can also help us to understand how species and communities affect ecosystem functioning. There are two different situations that should be addressed. First, different combinations of multiple traits can lead to the same effect on the environment (i.e. ecosystem processes or other species dynamics). The same reasoning discussed above for species response to environmental factors applies to species effects on ecosystem functioning. When the effect of an organism in the ecosystem is mediated by a trait measured at a high organism integration level, i.e. the effect trait (sensuLavorel and Garnier, 2002) is a whole-organism trait, different combinations of organ traits can result in the same species effect. For example, plant water transport is an important process in hydrological cycles, regulating water fluxes in watersheds and affecting local climate (Fisher et al., 2007; Asbjornsen et al., 2011). Sap flow rates are determined by a wide array of morphological, physiological and anatomical traits, such as leaf area, wood density, leaf area per stem cross-sectional area and stomatal control (Wullschleger et al., 1988; Wright et al., 2006; Apgaua et al., 2015). These traits are often not correlated at local scales, allowing similar sap flow rates as a result of different combinations of traits (O’Brien et al., 2004; Wright et al., 2006; Apgaua et al., 2015). Hence, dissimilar coexisting species can show convergent sap flow rates and therefore similar effects on hydrological processes.
Secondly, there is a good deal of evidence that many organ-level traits are directly related to species impacts on many ecosystem processes (Cornelissen, 1996; Cornelissen and Thompson, 1997; Díaz et al., 2004; de Bello et al., 2010). In this situation, the existence of alternative designs could result in high stability of ecosystem functioning when the loss of a species, for example, is compensated by another functionally different species but with comparable effects on the ecosystem. This is because environmental changes, with resulting filtering of species based on whole-organism traits, would not result in directional changes in organ-level (effect) traits. In this way, we propose alternative designs as a mechanism decoupling response (whole-organism) traits from effect (organ-level) traits. For instance, Rosado and de Mattos (2010, 2017) showed that dominance in plant communities on tropical sandy plains is determined by integrative traits related to plant water status during the dry season, such as mid-day plant water potential. The convergence of the co-dominant species on this integrative trait is the result of very different arrays of organ-level traits. In turn, the distinct arrays of organ traits, such as SLA, lead to contrasting effects on litter decomposition rates (Fig. 4). Additionally, the decoupling between traits and performance, as a result of alternative designs, can lead to redundancy between dominant and subordinate species regarding their contribution to ecosystem functioning. This would lead to high stability of ecosystem functioning as predicted by the insurance hypothesis (Elmqvist et al., 2003; Naeem and Wright, 2003). For instance, Walker et al. (1999) showed that functional similarity between dominant and minor species is responsible for the maintenance of ecosystem processes after changes in community composition due to increases in grazing intensity.
![Phytosociological importance value (IV) for four woody species in the Brazilian sandy coastal plains. Dominant species are represented by circles, and subordinate species are represented by squares. The dominance rank is explained by the minimum plant water potential (Ψ min, MPa), which is a whole-organism trait reflecting the integrated functioning of the plant. This integrative trait, in turn, is determined by distinct arrays of organ-level traits. For instance, dominant species show contrasting values of specific leaf area (SLA, cm2 g–1). This leaf trait is highly correlated with litter decomposition rate (k, year–1), which can be taken as a measure of the species impact on the ecosystem [data from Rosado and de Mattos (2010, 2018) and C. Dias (unpublished)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/aob/125/3/10.1093_aob_mcz180/1/m_mcz180f0004.jpeg?Expires=1750219721&Signature=JdHKSicr3B9BcZPI6sxVD01AliioMoDYNydsq3~UBKETXA4VMNP1DnH-yZwZTWZL4dM-elO2a6odLx6F56RXAEtmy9Q1ThnoLwlbAnpFfyGKMOrxzAPWi9dEgmVonwr7c66y~YmsVbzxwH3ubwxuAyD8vbuVdEjTP0CCw5a7q32V7RB3gjejCWgJ2zcT-o1TJOrIwQERWRg4fgTbVnedGyw-W~22bav5EQDSa1Wbmp4ZORpZWz15s88hlQA5S3rvgSW4FTVnr6OHcC2-9UJyYhSfS-kGzIekNYDjb2IuZqK50q8cgLOGHR-mJ6ELSZpnhk9Rixjc1MDNmVNGc1wZHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Phytosociological importance value (IV) for four woody species in the Brazilian sandy coastal plains. Dominant species are represented by circles, and subordinate species are represented by squares. The dominance rank is explained by the minimum plant water potential (Ψ min, MPa), which is a whole-organism trait reflecting the integrated functioning of the plant. This integrative trait, in turn, is determined by distinct arrays of organ-level traits. For instance, dominant species show contrasting values of specific leaf area (SLA, cm2 g–1). This leaf trait is highly correlated with litter decomposition rate (k, year–1), which can be taken as a measure of the species impact on the ecosystem [data from Rosado and de Mattos (2010, 2018) and C. Dias (unpublished)].
FUTURE CHALLENGES FOR INCORPORATING ALTERNATIVE DESIGNS IN COMMUNITY AND ECOSYSTEM ECOLOGY
As shown above, considering alternative designs can significantly contribute to our understanding of the community assembly process and its consequences for ecosystem functioning. To incorporate alternative designs in community and ecosystem ecology, three methodological aspects must be addressed. First, interactions between traits, i.e. accounting for the effects of different trait combinations, should be included when testing relationships between traits and performance. Boosted regression trees (BRTs) are a promising tool for such a task. Besides automatically accounting for complex non-linear relationships, BRTs use an algorithm to learn the relationship between the response variable and predictors, and fit interactions between predictors according to the size of trees used (Elith et al., 2008). Recently, Pistón et al. (2019) used BRTs to show that different trait combinations lead to similar demographical parameters between coexisting species. Additionally, they showed that BRTs outperform linear models when estimating the role of traits determining the relative importance of survival, growth and reproduction for population growth. Pearson et al. (2014) showed that BRTs were able to detect combinations of traits and population parameters that predict high extinction risks for amphibian and reptile species. In this way, BRTs can also be used to identify different trait combinations that result in high (or low) performance in a given environment (Pistón et al., 2019).
Secondly, models often used in trait-based ecology assume a monotonic relationship between traits and performance. Therefore, linear models are unable to detect complex relationships between traits and fitness as predicted by alternative designs. Recent advances propose alternative methods allowing testing of multimodal relationships between traits and fitness by using multimodal hierarchical Bayesian models (Laughlin et al., 2015) or calculating trait probability densities (Carmona et al., 2016).
Finally, the choice of traits should consider complex relationships of traits to performance and function, which are likely to depend on the trait integration level. It could be expected that performance will be chiefly determined by whole-organism traits (i.e. integrative traits), comprising morphological traits, such as size and architecture, but also physiological traits, such as growth rate and stress tolerance (Marks, 2007). Physiological traits are hard to measure in many species or in field conditions. These limitations can be overcome by ex situ controlled experiments that quantify whole-organism level performance such as stress survival (Sevanto et al., 2014). It is important to note that relationships between traits observed at a broad scale (e.g. the leaf economic spectrum, Wright et al., 2004; Díaz et al., 2016) are often not observed at local scales (Rosado and de Mattos, 2010; Wright and Sutton-Grier, 2012; Messier et al., 2017). Therefore, proxies for species response or species effects on ecosystem processes should be validated at the scale of the study (Rosado et al., 2013; Shipley et al., 2016). In this way, trait choice should account for the trait integration level and its implications for determining species performance and species effects on ecosystem processes (Marks, 2007; Rosado et al., 2013; Shipley et al., 2016). The combination of these methodological approaches will help in incorporating alternative designs into community and ecosystem ecology, allowing testing of how this widespread phenomenon influences community assembly and ecosystem functioning.
FUNDING
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. F.d.B. is funded by the Czech Science Foundation, grant P505/12/1296. F.d.B. and N.P. were supported by Brazilian CAPES PVE (grant no. 88881.068053/2014-01). N.P. is supported by a PNPD/CAPES grant. A.T.C.D. was supported by the Brazilian BJT [grant no. A011/2013 (Bolsista CAPES/BRASIL)] and CNPq grant [405579/2016-0]; B.H.P.R is supported by FAPERJ (Bolsa Jovem Cientista do Nosso Estado-JCNE, E-26/203.199/2016) and Prociência.
ACKNOWLEDGEMENTS
We thank Trude Schwarzacher and Martin Lechowicz for their constructive comments on this manuscript, and to Nichola S. Plowman for the helpful comments on the text.



