-
PDF
- Split View
-
Views
-
Cite
Cite
Mick E Hanley, Shareen K D Sanders, Hannah-Marie Stanton, Richard A Billington, Rich Boden, A pinch of salt: response of coastal grassland plants to simulated seawater inundation treatments, Annals of Botany, Volume 125, Issue 2, 23 January 2020, Pages 265–276, https://doi.org/10.1093/aob/mcz042
Close - Share Icon Share
Abstract
The combination of rising sea levels and increased storm frequency and intensity is predicted to increase the severity of oceanic storm surge events and the impact of flooding on coastal ecosystems globally. Understanding how plant communities respond to this threat necessitates experiments involving plant immersion in saline water, but logistical issues and natural variation in seawater composition mean that pure NaCl solutions or marine aquarium salts (MS) are widely used. Nonetheless, their comparative impact on plant ecophysiology, and thus relevance to understanding real-world flooding scenarios, is unknown.
In the first of two experiments, we examined how six ecophysiological responses in white clover (Trifolium repens) varied when plants were subjected to five different inundation treatments: deionized water, natural seawater, an MS solution and two NaCl solutions. In a second experiment, we examined how immersion in deionized water, MS solution and natural seawater affected six European perennial herb species, three native to Spanish sand dunes, and three from British coastal grasslands.
The two NaCl solutions induced exceptional Trifolium mortality, but responses varied little between MS and seawater treatments. In the second experiment, although leaf tissue necrosis and proline concentrations increased, and growth decreased compared with untreated controls, only one response in one species varied between MS and seawater treatments. Chemical speciation modelling revealed major variation in free Na+ and Cl− between NaCl solutions and seawater, but minor differences between MS and seawater.
We show that NaCl solutions are unsuitable surrogates to investigate plant response to elevated environmental salinity. Although responses to natural seawater and MS were consistent within species, there was notable between-species variation. Consequently, the first steps to elucidating how these species-specific responses influence coastal plant community recovery following storm surge can likely be achieved using commercial marine aquarium salts as substitutes for natural seawater.
INTRODUCTION
The past, present, and likely future impacts of anthropogenic climate change on plant species and communities are widely reported and reasonably well understood (Parmesan and Hanley, 2015). Most studies to date, however, focus on the long-term, chronic impacts of anthropogenic climate change (e.g. elevated CO2, variation in precipitation regimes and temperature increase), whereas much of the environmental threat is likely to stem from stressors and disturbances linked to an increased frequency and intensity of acute, extreme events (Rahmstorf and Coumou, 2011; Vasseur et al., 2014). Of these, coastal flooding represents one of the most significant challenges; a combination of increased sea-surface temperatures coupled with sea-level rise is predicted to increase the frequency and severity of oceanic storm surges globally (Vousdoukas et al., 2016; Vitousek et al., 2017). As a result, many low-lying coastal areas face an increased risk of seawater inundation (Nicholls and Cazenave, 2010) with supra-littoral habitats such as sand dunes, upper salt marshes and grasslands likely subject to periodic seawater immersion for the first time (Hoggart et al., 2014). Such habitats are both economically and ecologically important since they provide a natural sea defence and important refuge for many species excluded from intensive agriculture (Fisher et al., 2011; Duarte et al., 2013; Hanley et al., 2014). Consequently, understanding the response of coastal vegetation to any increase in the frequency and duration of seawater inundation is critical to ensuring effective coastal management (Hanley et al., 2014; Hoggart et al., 2014; Christie et al., 2018).
The impact of freshwater flooding on plants is well understood, but in addition to soil anoxia and reduced access to atmospheric O2 and CO2 (Colmer and Voesenek, 2009; Perata et al., 2011), seawater flooding imposes additional stresses. Most obviously, this is elevated salinity since seawater typically contains around 35 g L−1 (35 ‰) salt, of which chloride and sodium contribute 19 and 11 g L−1, respectively. Together, Na+ and Cl− cause both osmotic (limiting the plant’s ability to absorb water) and ionic (increased toxicity) stress, although for most species Na+ seems to exert more obvious (certainly better studied) toxic stress than Cl− (Maathuis and Amtmann, 1999; Munns and Tester, 2008). As noted by Kronzucker et al. (2013), this stress is widely associated with a detrimental shift in cytosolic K+/Na+ ratios and the disruption of cellular and whole-plant potassium homeostasis by Na+. As a general response, plants synthesize and accumulate stress metabolites (e.g. proline) and ions (i.e. K+) to exclude or compartmentalize Na+ and Cl− and re-establish homeostatic function (Flowers and Colmer, 2008; Munns and Tester, 2008). Even if successfully achieved, however, this likely imposes a cost on plant growth and reproductive potential (Munns and Tester, 2008; White et al., 2014; Hanley et al., 2019) with concomitant implications for subsequent population and community-level interactions. Understanding these ecophysiological and ecological responses to seawater inundation is consequently critical to understanding post-flooding community recovery, assembly and function (Tolliver et al., 1997; Tate and Battaglia, 2013; Hoggart et al., 2014; Lantz et al., 2015; Hanley et al., 2017).
Nonetheless, remarkably few studies have examined the response of coastal plant communities and their constituent species to acute seawater flooding, likely due in part to the difficulty in conducting realistic experiments. It is, for example, impossible to predict exactly where and when storm surges will occur and extremely unlikely that any two flooding events would be the same. As a result, our ability to examine the ‘before and after’ impacts of real-world flood events in the field is extremely limited (Middleton, 2009; Lantz et al., 2015). Similarly, manipulative field studies where supra-littoral coastal vegetation is experimentally flooded with seawater are rare (Tate and Battaglia, 2013); logistical and even ethical considerations are limiting. Even when achieved, most deliberately flooded sites experience long-term inundation over natural tidal cycles (Neubauer et al., 2013; Hopfensperger et al., 2014; Masselink et al., 2017), rather than acute, short-duration inundation of the kind experienced in the aftermath of storms. The lack of suitable field sites and scenarios necessitates a focus on controlled ‘flooding’ in laboratory and greenhouse experiments using locally collected seawater (Camprubi et al., 2012; Hanley et al., 2013, 2017; White et al., 2014). This raises a further issue, however, in that even if the ratio of the major elements remains ‘nearly constant’ (Levinton, 2001), there is marked seasonal and regional salinity variation in seawater (Dessier and Donguy, 1994; Donguy, 1994; Donguy and Meyers, 1996).
Given that the most significant impact of short-duration seawater immersion on plant metabolism and physiology seems to be associated with the effects of Na+ and Cl− (Flowers and Colmer, 2008; Munns and Tester, 2008), the simplest experimental approach would be to use a sodium chloride solution made up to typical seawater strength (i.e. 35‰) using deionized water. In addition to Cl− (±55 % of total chemical content) and Na+ (±31 %), however, seawater also contains the major ions SO42− (7.8 %), Mg2+ (3.7 %), Ca2+ (1.2 %) and K+ (1.1 %), and minor and trace elements (together <0.2 %), including bromine, carbon, strontium, boron, silicon, fluorine, nitrogen, phosphorus and iron (Levinton, 2001). The relative concentrations of many of these other elements are much more variable than those of Na+ and Cl− (Levinton, 2001; Wheeler et al., 2016) and their impact on plant metabolism and function less clear; some, e.g. K+, may have direct toxicological or osmotic effects while also having the potential to mitigate or amplify the impact of other elements (Flowers and Colmer, 2008).
One possible solution is to use commercially available marine aquarium salt compounds, which closely approximate typical inorganic chemical composition of seawater and offer a relatively consistent seawater surrogate (Flynn et al., 1995; Tolliver et al., 1997; Mopper et al., 2016). Nonetheless, some chemical seawater constituents (e.g. nitrogen and sulphur) are mobilized rapidly by biological processes and so their concentration is spatially and temporally variable (Levinton, 2001). Indeed, much of the solute content of seawater is derived from organic matter (living and dead), highlighting the important biological contribution to seawater chemistry (Levinton, 2001). This biological variability may impose additional impacts on terrestrial plant response to seawater inundation beyond the chemical effects alone.
The aim of this study was to elucidate how the response of common coastal plant species to simulated flooding varied according to the ‘seawater’ options available. Specifically, we test the hypothesis that the most commonly applied simulated seawater treatments all elicit similar plant physiological responses. In experiment 1 we subjected white clover (Trifolium repens) to immersion in (1) (deionized) water, (2) natural seawater, (3) commercially available marine aquarium salt, (4) sodium chloride solution balanced to average oceanic salinity (hereafter SalNaCl) and (5) sodium chloride solution balanced to the average ionic concentration of Instant Ocean (hereafter IonNaCl). We then examined subsequent mortality, plant growth, flowering and association with nitrogen-fixing bacteria to determine whether each treatment resulted in similar or varying plant responses. In experiment 2, we subjected six different coastal plant species to immersion in (1) deionized water, (2) natural seawater and (3) aquarium salt solution, quantifying immediate post-inundation proline accumulation and subsequent longer-term leaf necrosis and growth as measures of plant response.
MATERIALS AND METHODS
Plant collection and cultivation
Native to Europe, North Africa and Asia, white clover (Trifolium repens L., Fabaceae) is, by virtue of its value as a nitrogen-fixing pasture crop, now globally distributed. In its native range, however, it is a common component of coastal plant communities such as sand dunes, upper salt marshes and grasslands (Grime et al., 2007). In June 2011 we collected 12 large (±100 mm diameter), branched plant fragments with multiple rooting points from the upper section (700 m from a seawall) of a grassland pasture at South Efford Marsh near Aveton Gifford, Devon, UK (50°18′14″ N, 03°50′59″ W). All samples were taken from distinct patches separated by at least 5 m to reduce the likelihood of collecting material from the same individual (Ab-Shukor et al., 1988). The plant fragments were transplanted into 110 × 110 × 120 mm plastic pots containing John Innes No. 2 potting compost and cultivated in a sheltered outdoor area. See White et al. (2014) for full details.
In late summer 2016, we collected seeds of Centaurea nigra (Asteraceae), Lotus corniculatus (Fabaceae) and Plantago lanceolata (Plantaginaceae) from coastal grasslands located across southern England (Table 1). In late spring 2017 seeds of their congeners Centaurea polyacantha, Lotus creticus and Plantago coronopus were collected from sand dunes located near Zahara de los Atunes, Andalucía, Spain. Seeds of all species were collected from mature inflorescences of a minimum of 30 maternal plants, and, after drying and cleaning, stored in airtight containers at room temperature until germination.
Details of seed collection sites for six coastal dune and grassland species from SW Spain and southern England used to compare plant performance following simulated seawater flooding treatments
| Region . | Species . | Site name . | Latitude, longitude . |
|---|---|---|---|
| Southern England | Centaurea nigra L. | Saltash, Cornwall | 50°23′37″ N, 04°13′40″ W |
| Lotus corniculatus L. | Wembury, Devon | 50°18′59″ N, 04°06′14″ W | |
| Plantago lanceolata L. | Sandwich, Kent | 51°16′48″ N, 01°21′42″ E | |
| South-west Spain | Centaurea polyacantha Willd. | Atlanterra, Cadiz | 36°05′39″ N, 05°48′44″ W |
| Lotus creticus L. | Zahara, Cadiz | 36°08′15″ N, 05°51′01″ W | |
| Plantago coronopus L. | Zahara, Cadiz | 36°07′35″ N, 05°50′23″ W |
| Region . | Species . | Site name . | Latitude, longitude . |
|---|---|---|---|
| Southern England | Centaurea nigra L. | Saltash, Cornwall | 50°23′37″ N, 04°13′40″ W |
| Lotus corniculatus L. | Wembury, Devon | 50°18′59″ N, 04°06′14″ W | |
| Plantago lanceolata L. | Sandwich, Kent | 51°16′48″ N, 01°21′42″ E | |
| South-west Spain | Centaurea polyacantha Willd. | Atlanterra, Cadiz | 36°05′39″ N, 05°48′44″ W |
| Lotus creticus L. | Zahara, Cadiz | 36°08′15″ N, 05°51′01″ W | |
| Plantago coronopus L. | Zahara, Cadiz | 36°07′35″ N, 05°50′23″ W |
Details of seed collection sites for six coastal dune and grassland species from SW Spain and southern England used to compare plant performance following simulated seawater flooding treatments
| Region . | Species . | Site name . | Latitude, longitude . |
|---|---|---|---|
| Southern England | Centaurea nigra L. | Saltash, Cornwall | 50°23′37″ N, 04°13′40″ W |
| Lotus corniculatus L. | Wembury, Devon | 50°18′59″ N, 04°06′14″ W | |
| Plantago lanceolata L. | Sandwich, Kent | 51°16′48″ N, 01°21′42″ E | |
| South-west Spain | Centaurea polyacantha Willd. | Atlanterra, Cadiz | 36°05′39″ N, 05°48′44″ W |
| Lotus creticus L. | Zahara, Cadiz | 36°08′15″ N, 05°51′01″ W | |
| Plantago coronopus L. | Zahara, Cadiz | 36°07′35″ N, 05°50′23″ W |
| Region . | Species . | Site name . | Latitude, longitude . |
|---|---|---|---|
| Southern England | Centaurea nigra L. | Saltash, Cornwall | 50°23′37″ N, 04°13′40″ W |
| Lotus corniculatus L. | Wembury, Devon | 50°18′59″ N, 04°06′14″ W | |
| Plantago lanceolata L. | Sandwich, Kent | 51°16′48″ N, 01°21′42″ E | |
| South-west Spain | Centaurea polyacantha Willd. | Atlanterra, Cadiz | 36°05′39″ N, 05°48′44″ W |
| Lotus creticus L. | Zahara, Cadiz | 36°08′15″ N, 05°51′01″ W | |
| Plantago coronopus L. | Zahara, Cadiz | 36°07′35″ N, 05°50′23″ W |
Experiment 1
In early December 2014 stolon fragments of white clover (~10 mm long and with discernible roots) were cut from each of eight plants and used to cultivate 24 clones from each parent. Initially planted into 50-mm diameter pots containing John Innes No. 2 compost and retained in an unheated greenhouse with natural illumination (mean daily maximum 21.8 ± 0.7 °C, minimum 4.3 ± 0.3 °C), in March 2015 daughter ramets were transplanted into 75 × 75 × 80-mm plastic pots containing John Innes No. 2 compost. Plants were arranged randomly on trays with capillary matting (mean daily maximum 32.4 ± 1.1 °C, minimum 7.4 ± 0.3 °C), and watered twice weekly to pot capacity with tap water until the start of the experiment.
Experimental treatments
Class A volumetric glassware and glass-distilled deionized water (ddH2O) were used for preparation of all treatments to ensure reproducibility. Approximately 30 L of seawater was collected from Wembury, Devon, UK (50°19′03″ N, 04°05′03″ W) in mid-March and stored in large, sealed plastic containers outdoors in the dark for 74 d until used to reduce the pool of labile dissolved organic carbon compounds present. Conductivity at the time of use was 42.4 mS cm−1 and salinity 34.9 ‰. Aged seawater (hereafter SW) was one of our five main treatment groups, along with a no-salt immersion treatment of ddH2O (DW) and one using a commercially available marine aquarium salt (MS), Instant Ocean® (Aquarium Systems, Blacksburg, VA, USA). MS solutions using Instant Ocean have been used in studies on plant response to both saltwater flooding (Tolliver et al., 1997; Mopper et al., 2016) and increased soil salinity (Naumann et al., 2007, 2008), but its effects on plant growth and physiological responses have never been compared against natural seawater.
We dissolved 33.3 g L−1 of Instant Ocean in deionized water to achieve a salinity of 35.1 ‰. The balance of major cations (Na+ K+, Ca2+ Mg2+, Sr2+) and anions (Cl−, SO42−) in this MS closely approximates seawater salts, falling within 10 % of typical seawater concentrations by mole for most of the major anions and cations, but has 5-fold higher nitrate and 50-fold higher ammonium (Atkinson and Bingman, 1997). Many trace anions (e.g. Cu2+, Co2+) are also present at low (μm) level, although these variations relate only to total concentrations and do not take into account speciation, ion pair formation or actual bioaccessibility (Atkinson and Bingman, 1997). Different salts, however, exert variable ionic charges, such that saline solutions made up from different constituent salts can have the same salinity but different ionic strength. Consequently, we prepared two different sodium chloride solutions; one with the same salinity as typical seawater (SalNaCl) (Atkinson and Bingman 1997), the other with the same ionic strength (IonNaCl), based on Debye–Hückel theory (Debye and Hückel, 1923). We prepared 25.0 L of SalNaCl solution using trace metals grade (>99.99 %) sodium chloride (Sigma) in ddH2O to a final salinity of 35 ‰. A similar volume of IonNaCl was prepared with the same constituents, but assuming an average seawater ionic strength of 0.7 m (i.e. 38.7 g NaCl L−1 ddH2O). All ‘salt’ solutions, plus deionized water, were stored in sealed, dark plastic containers in the experimental greenhouse for 2 d prior to use for temperature equilibration.
In early June 2015, six established ramets were selected from each of the eight parent stock plants. Each ramet, uniform in size and appearance, was assigned at random to one of the five treatment groups, or a no-immersion control treatment. In so doing, we ensured that each treatment group received genetically identical material. Although seawater flooding following storms can persist for up to 96 h, a 24-h duration is typical for low-lying UK coastline habitats following tidal surge events (Environment Agency, 2014). By immersing to pot level (in large plastic tubs) we simulated short-term soil waterlogging; while we recognize that seawater inundation following storm-surge would likely result in shoot submergence, we were able to separate the effect of ionic imbalance in the root zone rather than the impact of oxygen deficiency caused by full immersion that our treatments would impose.
Immediately after immersion, pots were arranged randomly on a wire mesh-topped bench inside the greenhouse; the wire mesh allowed free drainage and prevention of cross-contamination between treatment groups. Forty-eight hours after immersion and thereafter every 2 d for a further 90 d, the pots were watered to capacity (with rain water). Mean maximum and minimum daily greenhouse temperatures during this phase of the experiment were 36.9 ± 0.8 and 13.2 ± 0.2 °C, respectively.
Post-immersion plant response and recovery
Following immersion, one randomly selected shoot on each plant was marked at a terminal node with loosely tied cotton thread (‘stolon growth’). This was used to quantify subsequent stolon elongation 35 d post-immersion, when we also estimated the proportion of above-ground necrotic tissue (‘necrosis’). Mortality was checked daily from the start to the end of the experiment 90 d post-immersion, when, after counting the number of fully matured inflorescences present, surviving plants were harvested (late August 2015). Plants were cleaned of any adhering compost before roots and shoots were separated and oven-dried at 50 °C for 24 h. Total dry weight biomass (roots and shoots combined) attained during the period after immersion was taken as a measure of plant growth. We also selected the longest root branch on each plant to quantify the number of rhizobia nodules per unit root length.
The effects of immersion treatment on necrosis and stolon growth at 35 d post-immersion and growth, flowering effort and nodules at harvest, were examined using one-way ANOVA; all data were logit [ln(x+1)]-transformed prior to analysis to ensure heterogeneity of variance, and Tukey pairwise comparisons used to locate differences between treatment means.
Experiment 2
In mid-June 2017, seeds of all six species were set to germinate in 225 mm × 165 mm × 50 mm (covered) propagator trays containing John Innes seed compost. One week after germination, 150 individual seedlings per species were transplanted into 50-mm diameter pots containing John Innes seed compost. All initial plant cultivation was conducted in a controlled growth room set at 15 °C and a 12-h day/night illumination regime. When the plants were 6 weeks old (early August), 150 individuals from each of the UK species were transplanted into 70 mm × 70 mm × 80 mm square pots containing John Innes seed compost and moved to an elevated, outdoor hard-standing area on the University of Plymouth campus. A similar procedure was used for the Spanish species, except that they were transplanted into horticultural sand (Westland Horticulture Ltd, Dungannon, UK) to better simulate sediment in their native sand dune habitat.
Experimental treatments
In early October 2017, 119 individual plants (checked for health and similar size) of each species were allocated at random to one of three treatment groups (DW, MS or SW), subdivided into 24- or 96-h immersion times, such that there were 17 replicate plants per treatment/immersion time combination, or a no-immersion control treatment,. Seawater was collected from Plymouth Sound, Devon, UK (50°19′03″ N, 04°05′03″ W) in October 2017; conductivity at the time of use was 41.6 mS cm−1 and salinity 34.0 ‰. The MS solution using Instant Ocean was prepared to a salinity of 34 ‰. Immediately after immersion, pots were arranged randomly on a wire mesh-topped bench inside a greenhouse.
Post-immersion proline accumulation
Seventy-two hours after immersion, five plants per treatment/immersion time group were selected at random for proline analysis. From these, fully expanded, healthy leaves were harvested and flash-frozen in liquid nitrogen before storage at −80 °C. Proline analysis was adapted from Shabnam et al. (2016). Briefly, ~ 50 mg of leaves were ground in 40 % v/v ethanol at a ratio of 20 µL mg−1 of leaf material in a cold pestle and mortar. The extract was stored at 4 °C overnight to allow extraction of proline before storage at −20°C. Proline standards or extract (50 µL) were heated with 100 µL of reaction mix (1.25 % w/v ninhydrin in glacial acetic acid) at 100 °C in a covered polypropylene 96-well plate for 30 min before centrifugation of the plate at 800g for 2 min. The supernatant fluid was transferred to clean plates and absorbances determined at 520 nm using an Omega Fluostar plate reader (BMG Labtech).
Post-immersion plant recovery
All remaining plants were cultivated for a further 100 d, with pots watered weekly to capacity with rainwater. Mean daily minimum and maximum greenhouse temperatures during this phase of the experiment were 6.1 ± 0.03 and 18.9 ± 0.06 °C, respectively. At 28 d post-immersion, we estimated the proportion of above-ground necrotic tissue (necrosis) present on each plant. Mortality was checked daily until the end of the experiment (early January 2018), when all surviving plants were harvested and processed as described above (experiment 1).
The effects of immersion treatment on proline, necrosis and growth were examined using one-way ANOVA on each species; all data were ln(x+1)-transformed prior to analysis and Tukey pairwise comparisons used to locate differences between treatment means. Due to the relatively large number of tests generated (i.e. six per response, three responses), we adopted P < 0.01 to avoid type I error.
Solute speciation modelling
Since the true levels of free ions, and ion pairs, in the solutions used here vary from the amounts of solute added (based on formation of ion pairs and precipitating minerals), it was necessary to model the chemical interactions within the solutions. In so doing, we were able to understand how the actual ion concentrations affected plants, rather than estimating effects from, for example, total sodium added. The speciation of ions, ion pairs and precipitates etc. was modelled using the MS composition given by Atkinson and Bingman (1997) and the SW composition given by Nordstrom et al. (1979). The PHREEQC Interactive 3.3.12 package (Parkhurst and Appelo, 1999) was used with the Lawrence Livermore National Laboratory database (llnl.dat), which is based on the EQ3/6 model of Wolery (1979). The model was run at 20 °C on the basis of 10 kg of solution under test with a headspace of 100 000 L of air comprising (% v/v) water vapour (1.00, since experiments were conducted ~1 km from the coast), carbon dioxide (0.04), oxygen (20.95), methane (0.00018), argon (0.93), neon (0.002) and helium (0.0005), balanced with nitrogen. Liquid and gas were at atmospheric pressure and the liquid was equilibrated with the headspace mixture.
RESULTS
Experiment 1
Plant mortality was exceptionally high in the IonNaCl and SalNaCl treatment groups, where all except one individual in SalNaCl died within 3 weeks of immersion. By contrast, no more than one plant died in any of the other treatment groups. As a result, all further analysis focused solely on the remaining DW, MS and SW treatment groups. At 35 d post-immersion, T. repens exhibited increased necrosis following MS or SW treatment (Fig. 1), but DW had no effect (F3,27 = 12.08, P < 0.001) compared with the no-immersion control. Stolon elongation, however, did not vary between treatment groups (F3,27 = 2.52, P = 0.079). By the end of the experiment, plants in both the MS and SW treatments were considerably smaller than untreated controls (F3,26 = 5.78, P = 0.004). Both flowering effort (F3,26 = 2.43, P=0.087) and root colonization by rhizobia (F3,26 = 2.14, P = 0.12) were unaffected by immersion treatment (Fig. 1). Post hoc examination of treatment means showed no variation in plant necrosis or final biomass between MS and SW treatments (Fig. 1).
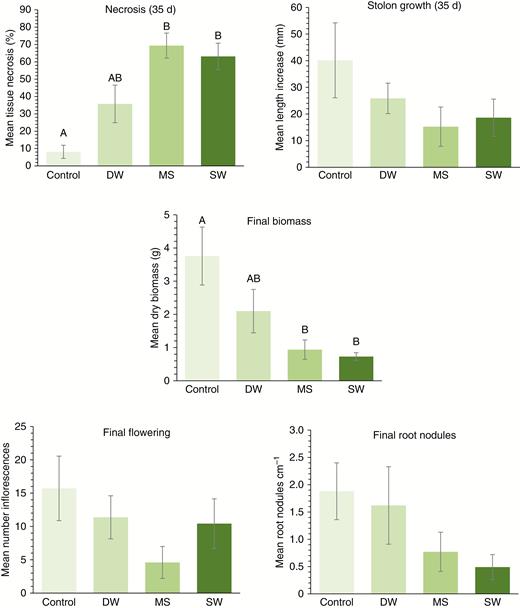
Responses of Trifolium repens to simulated seawater flooding (MS) and natural seawater (SW) compared with immersion in deionized water (DW) or untreated controls. Panels show effects on above-ground tissue necrosis and stolon extension at 35-d post-immersion, and final plant dry weight biomass, inflorescence number (per plant) and root colonization by rhizobia at 90 d post-immersion.
Experiment 2
No more than two plants of 12 in any of the species/treatment group combinations died over the course of the experiment and we attempt no further analysis on mortality. The effects of immersion treatments on initial proline accumulation varied between species and treatments (Fig. 2). For the two Centaurea species, although 96-h DW had no effect on leaf proline concentrations compared with untreated controls, the MS and SW immersion treatments resulted in significant accumulation (C. nigra, F5,24 = 22.6, P < 0.001; C. polyacantha, F6,28 = 4.45, P = 0.003). The effect was, however, more marked for C. nigra, where 96-h MS and SW immersion yielded a 3- and 5-fold, respectively, increase in proline concentrations (note that the 24-h DW sample for this species was lost prior to analysis). For C. polyacantha, post hoc analysis suggested that 24-h SW was the only treatment to stimulate significantly increased proline synthesis, even though concentrations more than doubled in all MS and SW treatments compared with the control. Lotus creticus (F6,28 = 5.43, P < 0.001) exhibited a similar response to C. nigra, i.e. higher proline levels in the longer MS and SW immersion treatments. Lotus corniculatus, however (F6,28 = 5.78, P < 0.001), had significantly increased proline concentrations only in 96-h MS and 96-h SW. Neither P. lanceolata (F5,28 = 1.65, P = 0.163) nor P. coronopus (F6,28 = 1.67, P = 0.165) showed any variation in post-immersion proline levels. Consistently for all species, we found no variation in proline accumulation response between time-equivalent MS or SW treatments.
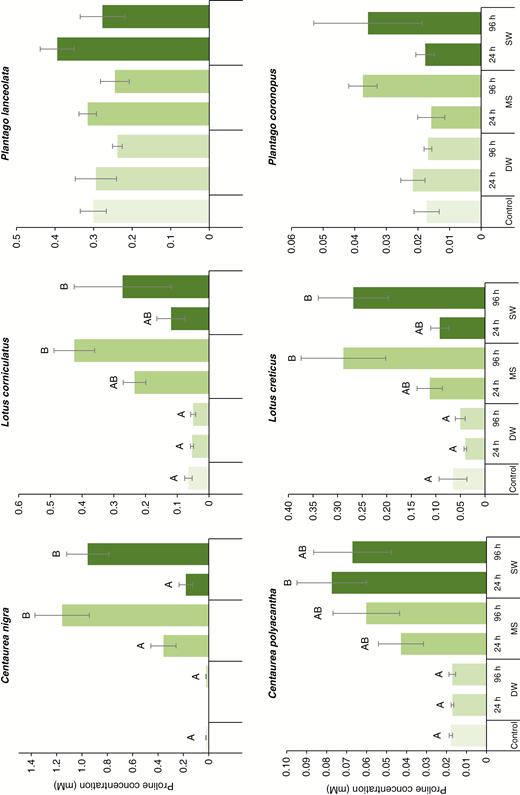
Effect of simulated seawater (MS), natural seawater (SW) and freshwater (DW) flooding on mean (± s.e.) leaf proline concentrations for six European coastal grassland species 3 d after root-zone immersion.
At 28 d post-immersion, all six species exhibited increased necrosis following MS or SW treatment (Fig. 3); DW had no effect. For Centaurea nigra (F6,77 = 13.01, P<0.001), 96-h MS and both SW treatments increased leaf necrosis compared with the control, while for C. polyacantha (F6,77 = 17.47, P < 0.001) all MS and SW treatments elicited this effect. Lotus corniculatus (F6,77 = 20.68, P < 0.001) was the only species exhibiting significant variation between time-equivalent (i.e. 24 h) MS and SW treatments, where 24-h SW did not vary from untreated controls. Although unaffected at shorter durations, L. creticus (F6,77 = 4.59, P = 0.001) displayed more necrosis in both 96-h MS and 96-h SW treatments. Both Plantago species suffered increased necrosis following MS and SW immersion; all treatments except 24-h MS caused increased necrosis in P. lanceolata (F6,77 = 7.97, P < 0.001), while for P. coronopus (F6,77 = 5.27, P < 0.001), elevated tissue necrosis was common throughout.
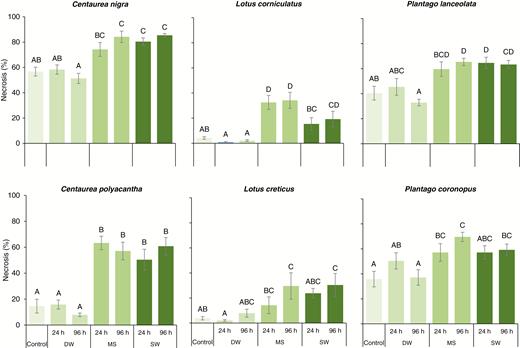
Effect of simulated seawater (MS), natural seawater (MS) and freshwater (DW) flooding on mean (± s.e.) above-ground tissue necrosis for six European coastal grassland species 28 d after root-zone immersion.
Five of the six species exhibited reduced growth (final plant dry biomass) following MS or SW treatment (Fig. 4); DW had no effect. For both C. nigra (F6,76 = 20.03, P < 0.001) and C. polyacantha (F6,74 = 16.74, P < 0.001), all MS and SW treatments resulted in reduced size. Plantago coronopus (F6,75 = 6.10, P < 0.001) exhibited a similar response, while P. lanceolata (F6,77 = 5.02, P < 0.001) plants in all MS and SW treatments except 96-h MS were smaller than controls. For the two Lotus species (L. corniculatus, F6,77 = 6.95, P < 0.001; L. creticus, F6,73 = 2.77, P = 0.018), however, we observed few treatment-specific effects; L. creticus did not achieve our P < 0.01 criterion, while for L. corniculatus, post hoc tests suggested that only plants in the 24-h MS treatment were smaller than controls. Nonetheless, consistently for all six species, there was no variation in final dry biomass between time-equivalent MS or SW treatments.
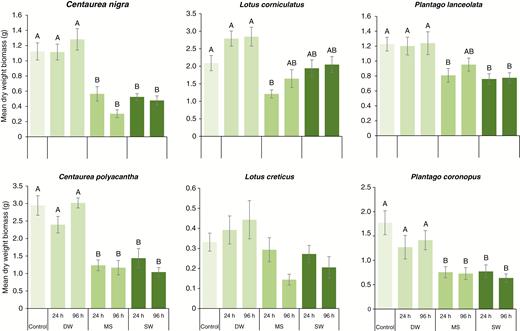
The effect of simulated seawater (MS), natural seawater (SW) and freshwater (DW) flooding on mean (±s.e.) total plant dry weight biomass for six European coastal grassland species 100 d after root-zone immersion.
Solute speciation modelling
Modelling of MS composition compared with SW showed that overall available Na+ and Cl− concentrations were broadly similar: MS, 430 and 488 mm, respectively; SW, 434 and 523 mm, respectively. For an NaCl solution that was salinity-matched to MS (i.e. SalNaCl), concentrations of free Na+ and Cl− were substantially higher (both 572 mm), with most of the ~25 mm that was not present as free ions (since 596 mm total Na+ and Cl− was added) found as the NaCl ion pair in solution. In both SW and MS, free K+ was present at 6.3 and 9.0 mm, respectively, a slight increase in the key ion used by plants to re-establish homeostatic function after exposure to NaCl (Munns and Tester 2008).
DISCUSSION
Our study presents three major conclusions. First, exceptionally high Trifolium mortality in the IonNaCl and SalNaCl treatments (experiment 1) shows that ‘pure’ NaCl solutions are unsuitable surrogates to study the effect of seawater immersion on plant physiology. Second, except one instance (necrosis in 24-h treatments for L. corniculatus), all MS versus SW comparisons suggest that a commercially available marine aquarium salt elicits similar plant ecophysiological responses to natural seawater. Finally, all species responded negatively to simulated seawater flooding (MS or SW treatments).
Although the greatest impact of seawater flooding on plant performance may stem from the ionic and osmotic stress imposed by Na+ and Cl−, our results suggest that other seawater constituents moderate these effects. From the methodological perspective, this is important because a number of studies have attempted to mimic the impact of salt-spray and/or seawater immersion using NaCl solutions applied directly onto the plant or soil surface (Ab-Shukor et al., 1988; Sykes and Wilson, 1989; van Puijenbroek et al., 2017; Varone et al., 2017). In so doing, these experiments fail to account for the likelihood that the ionic and osmotic stresses they ascribed to elevated Na+ and Cl− are in fact influenced or moderated by other salts. One area for further investigation (specifically in comparison with NaCl solutions) is to determine whether K+ in seawater (1.1 % of total salt concentration) helps mitigate deleterious changes in cytosolic K+/Na+ ratios and disruption of potassium homeostasis (Maathuis and Amtmann, 1999; Kronzucker et al., 2013). Similarly, changes in the cytoplasmic balance of Na+/SO42−, Na+/Mg2+ and Na+/Ca2+ ratios also have deleterious effects on plants grown in high salinity, effects likely magnified when ‘pure’ NaCl solutions are used rather than seawater, which naturally contains these SO42−, Mg2+ and Ca2+ ions (Maas and Grattan, 1999; Maathuis and Amtmann, 1999; Shabala et al., 2005). Our Trifolium response data (experiment 1) certainly call into question the biological relevance of the many studies that seek to assess crop plant response to increased soil salinity using NaCl solutions (e.g. Dai et al., 2018; Flam-Shepherd et al., 2018; Wu et al., 2018; Zhang et al., 2018). Salinized irrigation waters, for example, contain a range of cations and anions beyond Na+ and Cl− (Maas and Grattan, 1999) and our speciation modelling shows that a NaCl solution matched to average seawater salinity contains considerably more free Na and Cl ions than seawater (i.e. an increase in SalNaCl of 32 and 9 %, respectively).
Although commercial aquarium salts have been used to determine how salinity affects coastal plants (Tolliver et al., 1997; Mopper et al., 2004; Naumann et al., 2008), these studies have assumed, rather than demonstrated, that observed effects were compatible with those produced by natural seawater. Our results suggest that this assumption may be valid. In comparisons of six different biochemical, growth and reproductive responses involving seven different plant species, we found only one significant difference between time-equivalent SW and MS immersion treatments; i.e. above-ground tissue necrosis in L. corniculatus was twice the amount in 24-h MS immersion compared with 24-h SW plants. This necrosis response seems to have carried over into final plant biomass, where 24-h MS was the only treatment to display significantly reduced growth in comparison with the untreated control. The fact that these necrosis and biomass differences were not apparent in the 96-h treatments also suggests, however, that any response is at best short-lived and may even be a statistical artefact. The general consistency of observed biological responses corroborates our modelling of the compositions of MS and SW in that concentrations of free Na+ (<1 % difference) and Cl− (7 % higher in SW) ions are remarkably similar. Given its role in counteracting cytoplasmic Na+ accumulation, the (42 %) higher K+ availability in MS might suggest that plants subjected to MS rather than SW would recover better from simulated flooding. No plant response observed in our experiments corroborated this suggestion, however.
Although in experiment 2 all six species were affected negatively by (simulated) seawater immersion for at least two of the responses examined, there were some interesting patterns of response. First, and as might be expected, congenerics tended to react in broadly similar ways. For example, while neither Plantago species showed any variation in leaf proline concentrations, proline responses to all immersion treatments in the two Lotus species were remarkably similar. In Centaurea, necrosis and final plant biomass also showed very similar treatment-specific responses. More interesting than any indication of phylogenetic conservation was perhaps the general commonality of response of congenerics grown in different media (i.e. English species in commercial potting compost and Spanish species in horticultural sand). When coupled with the dramatic response of T. repens to SalNaCl and IonNaCl solutions in experiment 1, this observation suggests that achieving a field-relevant salinity treatment is a more important methodological consideration than what growing media is used to cultivate plants. Second, in terms of the overall lack of plant mortality, all species showed a remarkable tolerance to up to 4 d of simulated seawater flooding. Finally, the consistency of all other plant responses to MS and SW treatments nonetheless highlights the negative impact seawater flooding exerts on coastal vegetation, underscoring growing concerns about the predicted increase in the frequency and severity of oceanic storm surges on low-lying coastal areas (Nicholls and Cazenave, 2010).
An important consideration here is that all experiments were performed on plants grown in monoculture in greenhouse conditions, free from competition and environmental stressors. Indeed, even in controlled greenhouse experiments the responses of plants to simulated seawater flooding in monoculture changed when the same species were grown together (Hanley et al., 2017). Consequently, even apparently minor species-specific differences in plant response to seawater inundation are likely to be magnified in sand dunes, salt marshes and other coastal habitats following actual flood events, such that species composition is modified after the event (Engels and Jensen, 2010; Guo and Pennings, 2012; Schile et al., 2017). For example, a study on long-term tundra recovery following a major storm surge in the Canadian Arctic (Lantz et al., 2015) reported species-specific variation in plant recovery; specifically, graminoids exhibited greater resilience than shrubs. This is important because any reduction in species diversity or loss of key plant functional groups stemming from increased flood severity or frequency may reduce community resilience to further perturbation. Ford et al. (2016), for example, recently described how reductions in salt-marsh diversity led to increased erosion potential, particularly where sandy soils with low organic content predisposed these habitats to sediment loss. The global importance of plant communities to coastal defence, at a time when they also face increased flood risk (Duarte et al., 2013; Morris et al., 2018), gives urgency to our need to better understand how acute seawater inundation affects component species and ecosystem processes. Our inability to predict where and when flooding will happen and the difficulties associated with conducting manipulative experiments on natural communities mean that plant biologists may be constrained to work in more highly controlled systems to achieve this aim. We demonstrate here that although a pure NaCl solution is an inappropriate surrogate, commercial marine aquarium salts may offer a suitable alternative to the logistical problems and biochemical variations associated with using natural seawater.
ACKNOWLEDGEMENTS
We thank Martin Cooper, Tom Gove and Roberto Lo Monaco for technical assistance and two anonymous referees for their insightful comments on an earlier draft of the article.



