-
PDF
- Split View
-
Views
-
Cite
Cite
Xinyi He, Guoyin Liu, Bing Li, Yanwei Xie, Yunxie Wei, Sang Shang, Libo Tian, Haitao Shi, Functional analysis of the heterotrimeric NF-Y transcription factor complex in cassava disease resistance, Annals of Botany, Volume 124, Issue 7, 27 November 2019, Pages 1185–1197, https://doi.org/10.1093/aob/mcz115
Close - Share Icon Share
Abstract
The nuclear factor Y (NF-Y) transcription factor complex is important in plant growth, development and stress response. Information regarding this transcription factor complex is limited in cassava (Manihot esculenta). In this study, 15 MeNF-YAs, 21 MeNF-YBs and 15 MeNF-YCs were comprehensively characterized during plant defence.
Gene expression in MeNF-Ys was examined during interaction with the bacterial pathogen Xanthomonas axonopodis pv. manihotis (Xam). The yeast two-hybrid system was employed to investigate protein–protein interactions in the heterotrimeric NF-Y transcription factor complex. The in vivo roles of MeNF-Ys were revealed by virus-induced gene silencing (VIGS) in cassava.
The regulation of MeNF-Ys in response to Xam indicated their possible roles in response to cassava bacterial blight. Protein–protein interaction assays identified the heterotrimeric NF-Y transcription factor complex (MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12). Moreover, the members of the heterotrimeric NF-Y transcription factor complex were located in the cell nucleus and conferred transcriptional activation activity to the CCAAT motif. Notably, the heterotrimeric NF-Y transcription factor complex positively regulated plant disease resistance to Xam, confirmed by a disease phenotype in overexpressing plants in Nicotiana benthamiana and VIGS in cassava. Consistently, the heterotrimeric NF-Y transcription factor complex positively regulated the expression of pathogenesis-related genes (MePRs).
The NF-Y transcription factor complex (MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12) characterized here was shown to play a role in transcriptional activation of MePR promoters, contributing to the plant defence response in cassava.
Introduction
Cassava (Manihot esculenta) is widely distributed in tropical and subtropical regions and is the fourth largest crop after rice, sugarcane and corn. As a major source of carbohydrates and nutrients, cassava is an important staple food for 800 million people across the global tropical region (Howeler et al., 2013). Especially in the sub-Saharan Africa region, over 100 million tons of the cassava produced is consumed as food (Okogbenin et al., 2013). Cassava bacterial blight (CBB), caused by Xanthomonas axonopodis pv. manihotis, is one of the most devastating cassava diseases worldwide (Lopez et al., 2005; Munoz-Bodnar et al., 2014). This disease causes serious damage, affecting 12–100% of the crop in infected areas (Lozano, 1986; López and Bernal, 2012). Although a number of disease management strategies are employed for CBB, the identification of genes and crop improvement for host resistance offers considerable potential.
The NUCLEAR FACTOR Y (NF-Y) transcription factor complex, including NF-Y SUBUNIT A (NF-YA), NF-YB and NF-YC, is conserved in eukaryotes (Yazawa and Kamada, 2007; McNabb et al., 1995). NF-YB and NF-YC form a heterodimer in the cytoplasm and the dimer is translocated to the nucleus. The nuclear dimer then recruits NF-YA to generate the mature and heterotrimeric NF-Y transcription factor complex, which is required for CCAAT binding (Siefers et al., 2009; Petroni et al., 2012). To date, the NF-YA, NF-YB and NF-YC gene families have been identified across various plant species (Petroni et al., 2012), with each NF-Y subunit encoded by multiple genes. In Arabidopsis, for example, ten genes for each AtNF-Y subunit have been described (Petroni et al., 2012; Stephenson et al., 2007). The NF-Y complex is involved in gene transcriptional regulation during plant developmental processes and stress responses, including pollen tube development (von Besser et al., 2006; Yu et al., 2011), plant flowering (Ben-Naim et al., 2006; Wenkel et al., 2006; Chen et al., 2007; Ito et al., 2011; Hwang et al., 2016; Kim et al., 2016), root formation (Ballif et al., 2011), seed germination (Siriwardana et al., 2014; Ding et al., 2016; Liu et al., 2016), nodulation (Soyano et al., 2013; Baudin et al., 2015), grain yield (Qu et al., 2015; Yadav et al., 2015), endosperm development (Mei et al., 2015), stress response to low nitrogen (Li et al., 2015) and salt (Li et al., 2013; Ma et al., 2015a, b), drought tolerance (Alam et al., 2015; Chen et al., 2015; Lee et al., 2015; Palmeros-Suárez et al., 2015), response to cold (Shi et al., 2014), heat (Leyva-González et al., 2012; Sato et al., 2014) and disease resistance response (Alam et al., 2015; Rey et al., 2016).
Little is known about the role of NF-Y in plant immunity. NF-Y plays an important role in leguminous plant–rhizobium interaction. NF-YC1 interacts with SIN1 and specifically binds to the CCAAT motif to activate the expression of cell cycle-related genes as well as downstream genes, so as to regulate the infection of rhizobia and the formation of root nodules (Soyano et al., 2013, Battaglia et al., 2014, Rípodas et al., 2014). In Medicago truncatula, the MtNF-YA1/MtNF-YB16/MtNF-YC2 complex is involved in root nodule development through binding the promoter of Ethylene Responsive factor required for Nodulation (MtERN1) (Baudin et al., 2015). Rey et al. (2016) highlighted the dual role of MtNF-YA1 in susceptibility to a root pathogen through regulating several defence-related genes. Alam et al. (2015) found that the expression of OsHAP2E can be induced by salicylic acid, isonicotinic acid, abscisic acid, hydrogen peroxide (H2O2), Magnaporthe oryzae or Xanthomonas oryzae pv. oryzae. Moreover, overexpression of OsHAP2E confers resistance to M. oryzae and X. oryzae pv. oryzae in rice (Alam et al., 2015).
In addition to Arabidopsis, NF-YA, NF-YB and NF-YC have also been identified in multiple other plant species, including wheat (Triticum aestivum) (Stephenson et al., 2007), rice (Oryza sativa) (Alam et al., 2015), canola (Brassica napus) (Liang et al., 2014; Xu et al., 2014) and soybean (Glycine max) (Quach et al., 2015). In contrast, this transcription factor complex has been poorly characterized to date in cassava. In this study, 15 MeNF-YAs, 21 MeNF-YBs and 15 MeNF-YCs were identified. Additionally, expression profiling and functional analysis identified novel roles of MeNF-Ys in the defence response to CBB. The functional analysis of MeNF-Ys and the heterotrimeric NF-Y transcription factor complex offer potential in genetic breeding for disease-resistant cassava.
MATERIALS AND METHODS
Plant materials and growth conditions
South China 124 (SC124) plants were grown in soil under greenhouse conditions at an average temperature of 26 °C and with 12 h light (120–150 µmol quanta m−2 s−1 irradiance)/12 h dark cycles.
Genome-wide identification of MeNF-YAs, MeNF-YBs and MeNF-YCs
The candidate MeNF-YAs, MeNF-YBs and MeNF-YCs were identified in the Plant Transcription Factor Database (Jin et al., 2014) and the Pfam database (Finn et al., 2016). Multiple sequence alignments of NF-YAs, NF-YBs and NF-YCs were performed using Clustalx 1.83 and MEGA 5.05 (Tamura et al., 2011) based on the amino acid sequences corresponding to coding sequence (CDS). Additionally, the conserved motifs of MeNF-YAs, MeNF-YBs and MeNF-YCs were analysed using Multiple Em for Motif Elicitation v4.11.0.
RNA isolation and quantitative real-time PCR
RNA isolation, first-strand cDNA synthesis and quantitative real-time PCR were performed as described by Y. Wei et al. (2017). A large number of samples from at least ten leaf discs were collected for each experiment. In detail, fresh bacterial liquid cultures were diluted with 10 mm MgCl2 to an OD600 around 0.2. The bacterial liquid cultures were then syringe-infiltrated into cassava leaves. For the assay of gene expression in response to mock (10 mm MgCl2) or Xam infection, samples were taken at 0 h post-infiltration (hpi), 1 hpi, 3 hpi and 6 hpi. For virus-induced gene silencing (VIGS), samples were taken at 0 d post-infiltration (dpi), 1 dpi and 2 dpi after confirming that the expression of the target gene was inhibited. The relative expression levels were normalized to MeEF1a using the comparative 2−ΔΔCT method, with three repeats. Primers are listed in Supplementary Data Table S1.
Subcellular localization assay
To investigate the subcellular localization of MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12, the CDS of the genes was fused with green fluorescent protein (GFP) and transiently expressed in Nicotiana benthamiana leaves. The green fluorescent signals of GFP-MeNF-YA1/3, GFP-MeNF-YB11/16 and GFP-MeNF-YC11/12 were specifically co-localized with 4′,6-diamidino-2-phenylindole (DAPI)-stained cell nuclei.
Yeast two-hybrid analyses
The coding sequences of MeNF-YAs, MeNF-YBs and MeNF-YCs were cloned into the vectors of pGBKT7 and pGADT7. Primers with restriction enzyme sites are listed in Supplementary Data Table S2. Thereafter, the recombinant bait and prey plasmids were transformed into the yeast AH109 strain as described in the Matchmaker™ Gold yeast Two-Hybrid System user manual protocol (Clontech, Mountain View, CA, USA).
Bimolecular fluorescence complementation
MeNF-YAs, MeNF-YBs and MeNF-YCs were cloned into the pFGC-nYFP and pFGC-cYFP vectors (Kim et al., 2008). Primers are listed in Supplementary Data Table S2. The recombinant plasmids were then transformed into Agrobacterium GV3101. The GV3101 strains with the recombinant plasmids together with protein P19 were syringe-infiltrated into N. benthamiana leaves as described by Sparkes et al. (2006). At 2 dpi, DAPI-stained cell nuclei and yellow fluorescence were detected as described in Y. Wei et al. (2017).
Vector construction and expression in Nicotiana benthamiana
The coding sequences of MeNF-YAs, MeNF-YBs and MeNF-YCs were cloned into the pEGAD vector (35S::GFP) (Cutler et al., 2000). Primers are listed in Supplementary Data Table S2. The GV3101 strains with the recombinant plasmids together with P19 were again syringe-infiltrated into N. benthamiana leaves as described above. At 2 dpi, DAPI-stained cell nuclei and green fluorescence and were again detected as described above.
Transcriptional activation activity assay and luciferase (LUC) assay
For reporter vector construction, the sequences of 5×CCAAT-mini35S, 5×CATAT-mini35S and promoters of MePRs were cloned into the pGreenII0800-LUC vector. Primers are listed in Supplementary Data Table S2. The effector plasmid (pEGAD or MeNF-Ys-pEGAD) and reporter plasmids (5×CCAAT-mini35S-pGreenII0800-LUC, 5×CATAT-mini35S-pGreenII0800-LUC and pMePRs-pGreenII0800-LUC) were co-transformed into cassava leaf protoplasts as described by Y. Wei et al. (2017, 2018). Thereafter, the relative LUC was quantified using a Dual Luciferase Reporter Gene Assay Kit (Beyotime, RG027, China).
VIGS in cassava
The partial coding sequences of MeNF-YAs, MeNF-YBs and MeNF-YCs were cloned into the pTRV2 vector (Liu et al., 2002). Primers are listed in Supplementary Data Table S2. The GV3101 strains with MeNF-Ys-pTRV2, pTRV1 and P19 were then syringe-infiltrated into cassava leaves as described by Sparkes et al. (2006).
Data analysis
Average values and standard deviations are presented in this study. Significance was tested with Student’s t-test and Duncan’s range test using Statistical Analysis System (SAS) v9.4 software.
RESULTS
Genome-wide identification of MeNF-YAs, MeNF-YBs and MeNF-YCs in cassava
A total of 15 MeNF-YAs, 21 MeNF-YBs and 15 MeNF-YCs were identified in the cassava genome (Supplementary Data Table S3). Based on phylogenetic analysis, MeNF-YAs could be divided into four clades, with the similarities of MeNF-YA5/7/8, MeNF-YA2/4 and MeNF-YA6/10 being relatively high (Fig. 1A). MeNF-YBs and MeNF-YCs could be divided into five and six clades, respectively (Fig. 1B), with the greatest similarity observed among MeNF-YC1/2/3 (Fig. 1C). Conserved domains in MeNF-Ys (Supplementary Data Fig. S1) and the evolutionary relationships of NF-Y members between cassava and Arabidopsis thaliana or O. sativa (Fig. 1) were analysed, revealing conservation in MeNF-Ys and clustering with homologues of dicotyledonous and monocotyledonous plants.
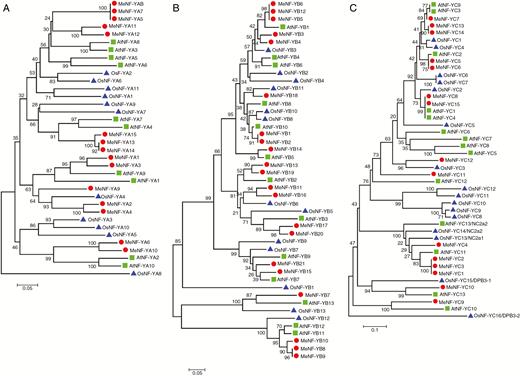
Neighbour-joining phylogenetic tree of 15 MeNF-YAs (A), 21 MeNF-YBs (B) and 15 MeNF-YCs (C). The sequences of amino acids corresponding to CDS were used for alignment analysis. The numbers and the scale bars indicate the relative values of phylogenetic relationship.
Expression profiles of MeNF-YAs, MeNF-YBs and MeNF-YCs in response to Xanthomonas axonopodis pv. manihotis (Xam)
In order to determine a possible involvement of MeNF-Ys in the plant immune response, the transcriptional profiles of MeNF-YAs, MeNF-YBs and MeNF-YCs in response to Xam were analysed by quantitative real-time PCR. For MeNF-YAs, expression of MeNF-YA6 and MeNF-YA15 peaked at 1 h, while the expression of MeNF-YA1 and MeNF-YA5 peaked at 6 h (Fig. 2A). For MeNF-YBs, expression of MeNF-YB7, MeNF-YB11, MeNF-YB16, MeNF-YB18 and MeNF-YB21 peaked at 6 h, while expression of MeNF-YB1-6, MeNF-YB8-10, MeNF-YB12-15 and MeNF-YB19-20 peaked at 1 h (Fig. 2B). For MeNF-YCs, expression peaked at 1 h, with the exception of MeNF-YC12 (6 h) (Fig. 2C). Based on expression profiles, MeNF-YA1, MeNF-YA3, MeNF-YB11, MeNF-YB16 and MeNF-YC12 were selected for further analysis.
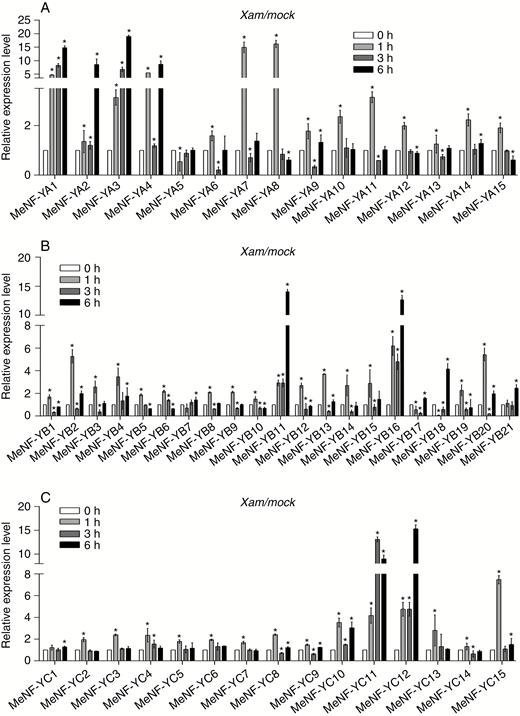
Expression profiles of (A) MeNF-YAs, (B) MeNF-YBs and (C) MeNF-YCs in response to Xam treatment. For treatment, leaves of 30-d-old plants were syringe-infiltrated with 10 mm MgCl2 (mock) or 108 cfu mL−1 of Xam in 10 mm MgCl2 for 0, 1, 3 or 6 h. The error bars represent SDs. *P < 0.05 compared with mock treatment.
Subcellular localization of MeNF-YAs, MeNF-YBs and MeNF-YCs
In analysis of tobacco leaves with the target genes (MeNF-YA1, MeNF-YA3, MeNF-YB11, MeNF-YB16, MeNF-YC11 and MeNF-YC12), green fluorescence only appeared in the nucleus of epidermal cells. Protein localization of MeNF-YA1, MeNF-YA3, MeNF-YB11, MeNF-YB16, MeNF-YC11 and MeNF-YC12 was observed in the nucleus according to the position of green fluorescence (Fig. 3).
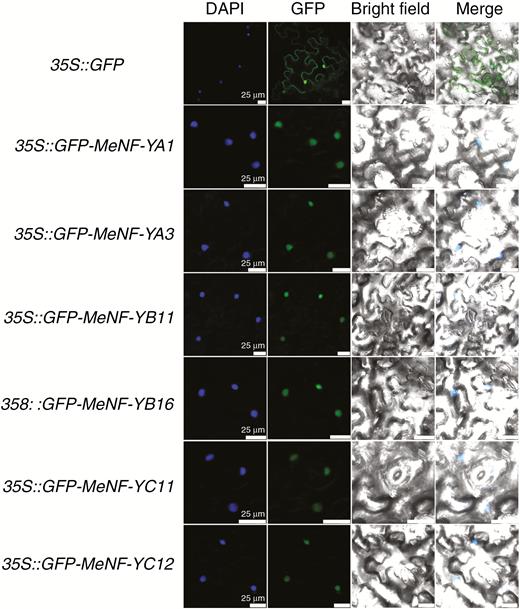
Subcellular localization of MeNF-YAs, MeNF-YBs and MeNF-YCs. Green fluorescence of 35S::GFP and 35S::GFP-MeNF-Ys was expressed in N. benthamiana leaves. DAPI-stained cell nuclei and green fluorescence are shown. Scale bars = 25 μm.
Heterotrimeric NF-Y transcription factor complex
NF-YA, NF-YB and NF-YC are three unique subunits of the NF-Y transcription factor complex. NF-YB and NF-YC form a heterodimer, which recruits NF-YA to generate a mature and heterotrimeric NF-Y transcription factor complex. To investigate the heterotrimeric NF-Y transcription factor complex between MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12, yeast two-hybrid assays were performed. The growth of positive clones on selective SD medium (SD-Leu-Trp-His-Ade) indicated that MeNF-YA1/3 and MeNF-YC11/12 interact with MeNF-YB11/16, while MeNF-YA1 interacts with MeNF-YC11 (Fig. 4A). Negative controls are shown in Supplementary Data Fig. S2. Consistently, the bimolecular fluorescence complementation (BiFC) assay confirmed the protein interaction between MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12 in planta (Fig. 4B). Although the interaction between MeNF-YC11 and MeNF-YA-3 and the interaction between MeNY-YC12 and MeNF-YA1/3 were not examined in yeast two-hybrid assays, interactions were observed in the BiFC assay (Fig. 4A, B). Thus, in vitro and in vivo assays showed the heterotrimeric NF-Y transcription factor complex in cassava (MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12).
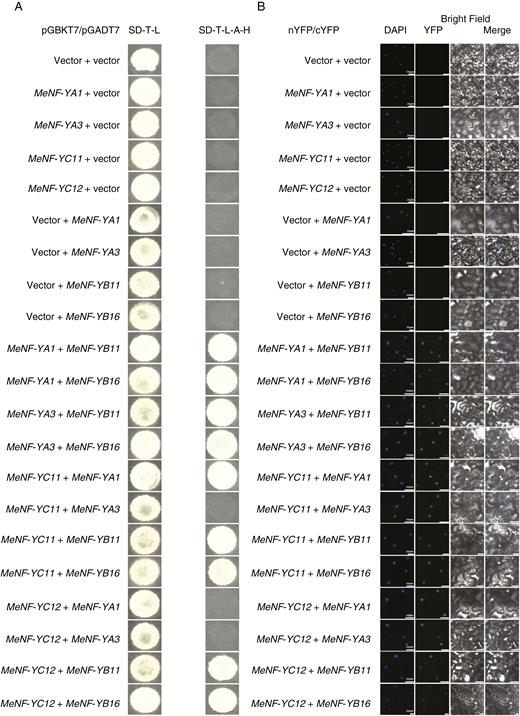
Protein interaction between MeNF-YAs, MeNF-YBs and MeNF-YCs. (A) Yeast two-hybrid assay showing the physical interaction between MeNF-YAs, MeNF-YBs and MeNF-YCs. The positive clones of interacted proteins were selected on selective SD medium (SD-Leu-Trp-His-Ade). (B) BiFC assay showing protein interaction between MeNF-YAs, MeNF-YBs and MeNF-YCs in planta. DAPI-stained cell nuclei and yellow fluorescent protein (YFP) are shown. Scale bars = 25 μm.
Transcriptional activation activity of the heterotrimeric NF-Y transcription factor complex
As CCAAT can be recognized by plant NF-Y transcription factors (Petroni et al., 2012), the dual LUC assay was performed in cassava leaf protoplasts to investigate the effect of MeNF-Ys on the activity of 5×CCAAT-mini35S. Overexpression of MeNF-YA1/3, MeNF-YB11/16 or MeNF-YC11/12 slightly increased the LUC activity of 5×CCAAT-mini35S-pGreenII0800-LUC in leaf protoplasts (Fig. 5). When these proteins were co-overexpressed, LUC activity was much higher than that observed through overexpression of MeNF-YA1/3, MeNF-YB11/16 or MeNF-YC11/12 (Fig. 5). Whilst single proteins were the least effective in activating the CCAAT box, certain double proteins did not increase activation above that of single proteins, such as MeNF-YA3+YC11 and MeNF-YB16+YC11. Notably, the ability of the triple proteins to activate the CCAAT box was greater than that observed for the single or double proteins. However, overexpression of these proteins had no significant effect on LUC activity of 5×CATAT-mini35S-pGreenII0800-LUC in cassava leaf protoplasts (Fig. 5). In summary, the NF-Y transcription factor complex conferred CCAAT binding and transcriptional activation in cassava.
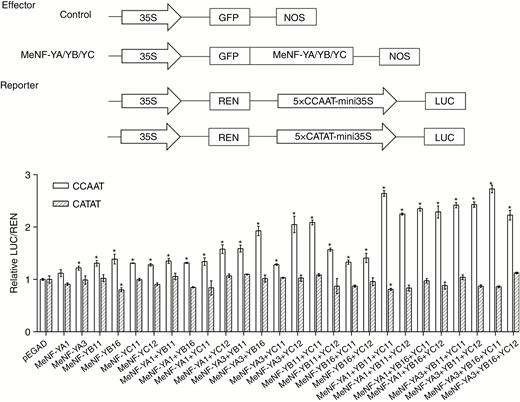
Transcriptional activation activity of the heterotrimeric NF-Y transcription factor complex. For the assay, pEGAD and MeNF-Ys-pEGAD were used as the effectors and 5×CCAAT-mini35S-pGreenII0800-LUC and 5×CATAT-mini35S-pGreenII0800-LUC were used as the reporters. *P < 0.05 compared with mock treatment. NOS, nopalinesynthase; REN, renilla.
Heterotrimeric NF-Y transcription factor complex regulates plant disease resistance
In order to examine the functions of MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12 in plant disease resistance to CBB, heterologous overexpression of the genes was performed in tobacco and homologous inhibition in cassava. According to bacterial propagation in plant leaves, overexpression of MeNF-YA1/3, MeNF-YB11/16 or MeNF-YC11/12 increased plant disease resistance (Fig. 6). In addition, gene-silenced plants with MeNF-YA1/3, MeNF-YB11/16 or MeNF-YC11/12, with viral expression of TRV1/TRV2 and lower expression of corresponding genes by VIGS (Fig. 7A, B), displayed greater bacterial propagation in leaves, indicating decreased disease resistance (Fig. 8A, B). Moreover, plants with MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12 co-silencing showed considerably more bacterial propagation in the leaves than control plants (Fig. 8A, B). These results indicate that the heterotrimeric NF-Y transcription factor complex positively regulates plant disease resistance to Xam.
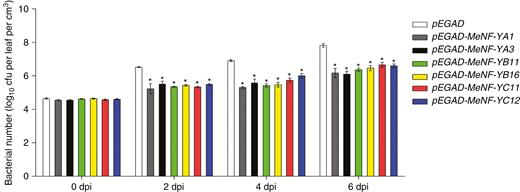
Overexpression of MeNF-Ys confers improved disease resistance to Xam in N. benthamiana. GV3101 strains together with P19 were syringe-infiltrated into N. benthamiana leaves for 2 d, and 108 cfu mL−1 of Xam in 10 mM MgCl2 was syringe-infiltrated for the disease resistance assay. *P < 0.05 compared with mock treatment.
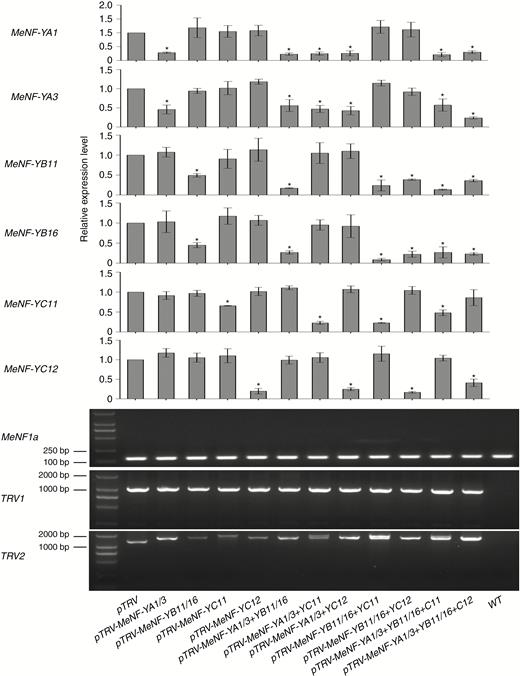
Expression levels of MeNF-Ys in plants with MeNF-Y silenced by VIGS. Expression of TRV1 and TRV2 in systemic leaves after pTRV1 and pTRV2 syringe-infiltration. PCR was performed using specific primers for the reference gene MeEF1a and viral expression of TRV1/TRV2. At 14 dpi of VIGS, corresponding gene expression was examined, and 108 cfu mL−1 of Xam in 10 mm MgCl2 was syringe-infiltrated for the disease resistance assay. The error bars represent SDs. *P < 0.05 compared with mock treatment.
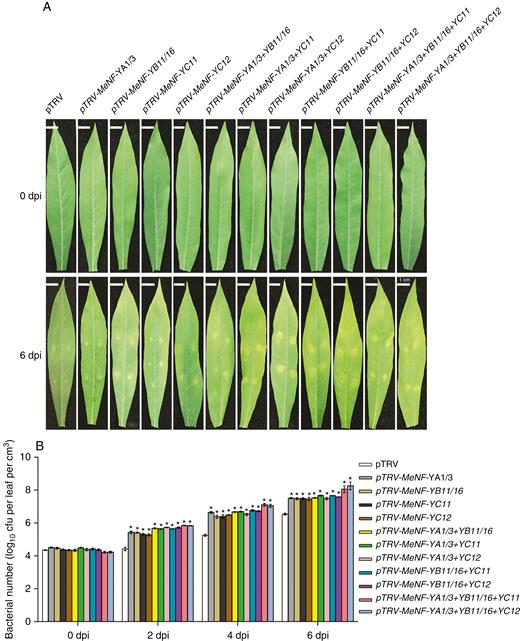
MeNF-Y-silenced plants show susceptibility to Xam disease. (A) Resistance of gene-silenced plants to Xam disease. (B) Number of Xam bacteria in plant leaves. *P < 0.05 compared with mock treatment.
Heterotrimeric NF-Y transcription factor complex regulates the expression of pathogenesis-related genes
Promoter analysis in pathogenesis-related genes (MePRs), which are important components in the plant defence responses (Wei et al., 2018), revealed several CCAAT motifs (Fig. 9A). Expression levels and promoter activities of MePRs were further investigated. In gene-silenced MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12 plants and MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12 co-silenced plants, the expression levels of MePR1, MePR2, MePR3 and MePR4 were significantly lower than those observed in the control plants (Fig. 9B). Consistently, the dual LUC assay showed that overexpression of MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12 significantly activated the promoter activities of MePRs in cassava leaf protoplasts (Fig. 9C).
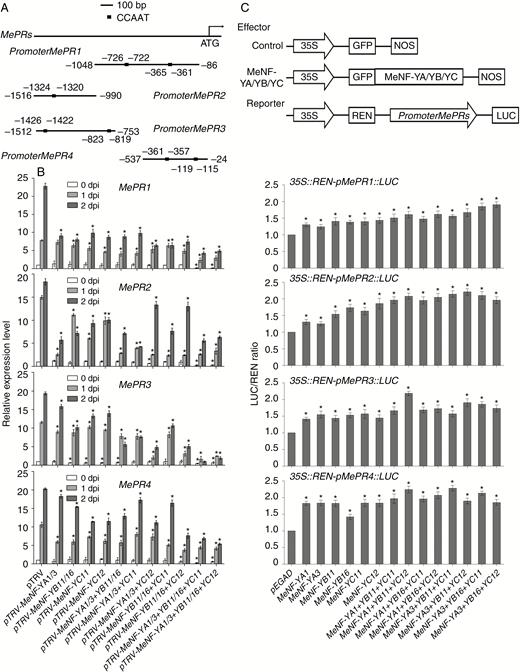
Heterotrimeric NF-Y transcription factor complex regulates the expression of MePRs. (A) Schematic structure of promoters of MePRs with CCAAT motifs. (B) Transcript levels of MePRs in plants gene-silenced by VIGS. (C) Effect of heterotrimeric NF-Y transcription factor complex on promoter activity of MePRs in cassava leaf protoplasts. *P < 0.05 compared with mock treatment. NOS, nopalinesynthase; REN, renilla.
Discussion
Protein interactions between NF-Ys have been described in various plant species. In A. thaliana, for example, AtNF-YA5/AtNF-YB9/AtNF-YC9, AtbZIP28/AtNF-YB3/AtNF-YC2/AtNF-YA4 and AtNF-YA2/AtNF-YB3/AtNF-YC10 complexes are involved in the regulation of chlorophyll A/B binding protein genes in etiolated seedlings, in the endoplasmic reticulum stress response and in heat stress-related gene expression, respectively (Warpeha et al., 2007; Liu and Howell, 2010; Sato et al., 2014). In rice, OsNF-YB1 interacts with OsNF-YCs and OsERF115 to regulate grain filling and endosperm development (Xu et al., 2016). In wheat, the TaNF-YC15/TaNF-YB2/4 complex is involved in drought tolerance (Yadav et al., 2015). In maize, ZmNF-YB16 improves drought responses by modulating the expression of photosynthesis- and antioxidant-related genes (Wang et al., 2018). In chrysanthemum, CmNF-YB8 bound to the promoter of the CmMIR156 gene is involved in the control of flowering (Q. Wei et al., 2017).
Although NF-Ys have been widely reported in other plants, information regarding the gene family and their possible role in plant defence responses in cassava remains unclear. In this study, 15 MeNF-YAs, 21 MeNF-YBs and 15 MeNF-YCs were identified, with multiple conserved motifs. Consistently, the neighbour-joining phylogenetic analysis of MeNF-YAs, MeNF-YBs and MeNF-YCs confirmed the phylogenetic relationship of NY-Ys in cassava, Arabidopsis and rice. Gene expression analysis highlighted common regulation of MeNF-Ys and expression induction by Xam, the predominant pathogen of CBB, suggesting a possible involvement of MeNF-Ys in plant immunity. Based on the expression data, certain MeNF-YAs, MeNF-YBs and MeNF-YCs were cloned for further analysis. As observed in other plant species (Siefers et al., 2009; Petroni et al., 2012), MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12 formed a heterotrimeric NF-Y transcription factor complex. Moreover, MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12 were located in the cell nucleus, with transcription activity on the CCAAT motif in cassava leaf protoplasts, indicating that they are transcription factors. Although Petroni et al. (2012) showed that the mature NF-Y transcription factor complex is required for CCAAT binding, Shi et al. (2014) found that overexpression of single AtHAP5A activated CCAAT motifs. Herein, overexpression MeNF-YA1/3, MeNF-YB11/16 or MeNF-YC11/12 significantly affected the promoter activity of 5×CCAAT-mini35S-pGreenII0800-LUC in cassava leaf protoplasts, with the co-overexpression of the components leading to higher activity.
Although N. benthamiana is not the native host of Xam, its leaves can be infected by Xam, resulting in disease symptoms and leaf bacterial population proliferation (Y. Wei et al., 2017). Overexpression of MeNF-YA1/3, MeNF-YB11/16 or MeNF-YC11/12 led to increased disease resistance against Xam in N. benthamiana, similar to the observed enhancement of resistance by overexpression of OsHAP2E in plants to M. oryzae and X. oryzae pv. oryzae in rice (Alam et al., 2015). MeNF-Ys may therefore be positive regulators of disease resistance in cassava. In addition, silencing of MeNF-YA1/3, MeNF-YB11/16 or MeNF-YC11/12 through VIGS resulted in increased disease susceptibility to Xam, with plants having MeNF-YA1/3-MeNF-YB11/16-MeNF-YC11/12 co-silencing displaying greater sensitivity. Taken together, the data indicate that the mature NF-Y transcription factor complex (MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12) may be essential for plant defence responses in cassava.
The MeNF-Y transcription factors may be involved in regulating plant disease resistance response through the proposed mechanism illustrated in Fig. 10. MeNF-YAs, MeNF-YBs and MeNF-YCs form the heterotrimeric NF-Y transcription factor complex, potentially binding to CCAAT motifs in the promoter regions of multiple downstream genes, including MePRs. Herein, heterotrimeric NF-Y transcription factor complex-silenced plants showed lower transcript levels of MePRs. Since CCAAT motifs are distributed in the promoter regions of MePRs, MeNF-Ys are speculated to be the upstream transcription factors of the MePRs. In accordance with previous studies (Alam et al., 2015; Rey et al., 2016), MeNF-Ys also positively regulate plant disease resistance. Comparative transcriptome analysis has identified 1509 genes differentially expressed by MtNF-YA1, including several defence-related genes and genes in hormone pathways (Rey et al., 2016). Based on RNA-seq data, some genes in hormone pathways also appear to be possible targets of MeNF-Ys. Considering the common regulation of OsHAP2E by hormones and the effects of MeNF-Ys on the expression of MePRs, the link between plant NF-Ys, defence-related genes and plant hormones might be directly related to the NF-Y-mediated plant disease response. Further studies into cloning MePR promoters and identification of MeNF-Y targets could provide more clues about the mechanism of MeNF-Y-mediated defence responses in cassava.
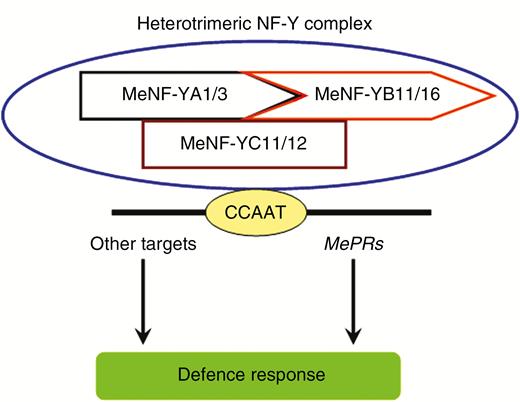
A possible model of cassava heterotrimeric NF-Y transcription factor complex in plant disease resistance.
In summary, transcriptional activity of the NF-Y transcription factor complex in cassava, formed by MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12, was shown to play a role in transcriptional activation of MePR promoters, contributing to the plant defence response.
SUPPLEMENTARY DATA
Supplementary data are available online at https://dbpia.nl.go.kr/aob and consist of the following. Fig. S1: analysis of conserved motifs of 15 MeNF-YAs (A), 21 MeNF-YBs (B) and 15 MeNF-YCs (C). Fig. S2: negative controls for MeNF-YA1/3, MeNF-YB11/16 and MeNF-YC11/12 with other MeNF-YA/B/C employed in yeast two-hybrid experiments. Table S1: primers employed in quantitative real time-PCR. Table S2: primers employed in vector construction. Table S3: genome-wide identification of 15 MeNF-YAs, 21 MeNF-YBs and 15 MeNF-YCs.
Funding
This research was supported by the National Natural Science Foundation of China (No. 31760067), startup funding and the scientific research foundation of Hainan University (No. kyqd1531) and the crop science postgraduate innovation project of Hainan University Tropical Agriculture and Forestry College (No. ZWCX2018017 and No. ZWCX2018018).
ACKNOWLEDGEMENTS
We thank Dr Xin Wang and Dr Wen Liu for their help in revising the manuscript.



