-
PDF
- Split View
-
Views
-
Cite
Cite
Fernanda Gomes Rodrigues, Néstor Franco Marulanda, Saura R Silva, Bartosz J Płachno, Lubomír Adamec, Vitor F O Miranda, Phylogeny of the ‘orchid-like’ bladderworts (gen. Utricularia sect. Orchidioides and Iperua: Lentibulariaceae) with remarks on the stolon–tuber system, Annals of Botany, Volume 120, Issue 5, November 2017, Pages 709–723, https://doi.org/10.1093/aob/mcx056
Close - Share Icon Share
Abstract
Background and Aims The ‘orchid-like’ bladderworts (Utricularia) comprise 15 species separated into two sections: Orchidioides and Iperua. These robust and mostly epiphytic species were originally grouped within the section Orchidioides by the first taxonomical systems. These species were later split into two sections when sect. Iperua was proposed. Due to the lack of strong evidence based on a robust phylogenetic perspective, this study presents a phylogenetic proposal based on four different DNA sequences (plastid and nuclear) and morphology to test the monophyly of the two sections.
Methods In comparison with all previous phylogenetic studies, the largest number of species across the sections was covered: 11 species from sections Orchidioides and Iperua with 14 species as an external group. Maximum likelihood and Bayesian inferences were applied to DNA sequences of rps16, trnL-F, matK, the internal transcribed spacer (ITS) and three morphological characters: (1) the crest of the corolla; (2) the primary organs in the embryo; and (3) tubers. Additionally, a histochemical analysis of the stolons and tubers is presented from an evolutionary perspective.
Key Results The analyses showed the paraphyly of sect. Iperua, since Utricularia humboldtii is more related to the clade of sect. Orchidioides. Utricularia cornigera is grouped in the sect. Iperua clade based on chloroplast DNA sequences, but it is nested to sect. Orchidioides according to ITS dataset. Morphological characters do not support the breaking up of the ‘orchid-like’ species into two sections, either. Moreover, the stolon–tuber systems of both sections serve exclusively for water storage, according to histological analyses.
Conclusions This study provides strong evidence, based on DNA sequences from two genomic compartments (plastid and nucleus) and morphology to group the Utricularia sect. Orchidioides into the sect. Iperua. The tubers are important adaptations for water storage and have been derived from stolons at least twice in the phylogenetic history of ‘orchid-like’ bladderworts.
INTRODUCTION
Lentibulariaceae is a cosmopolitan family presenting the greatest diversity in species, habit and life form among carnivorous plants. Around 350 species are distributed across the three genera Pinguicula, Genlisea and Utricularia; Pinguicula is the sister group of the clade formed by Genlisea and Utricularia (Jobson et al., 2003; Müller et al., 2004; Guisande et al., 2007; Veleba et al., 2014). The genus Utricularia forms traps from little vesicles called utricles or bladders, which have an active suction mechanism triggered when the trichomes near their entrance are stimulated by small organisms (Poppinga et al., 2016). Based on the vegetative morphology, Taylor (1989) split the genus Utricularia in into two subgenera: Polypompholyx and Utricularia. Nevertheless there are interesting, controversial proposals regarding the classification of the two very close infrageneric taxa: sections Orchidioides and Iperua.
The nine species of Utricularia sect. Orchidioides A.DC. are distributed in central America, the Antilles and South America and are orchid-like bladderworts (Fig. 1A-B, F). Moreover, they are perennial epiphytes or terrestrials, with a tuber ensemble in the peduncle basis (Fig. 9B, C). On the other hand, Utricularia sect. Iperua P.Taylor has six species distributed in South America (Fig. 1C-E, G). These are lithophytes and terrestrial or aquatic epiphytes (U. nelumbifolia and U. humboldtii are examples of the latter) and the majority of them form fleshy stolons (Fig. 9A), except for U. geminiloba, which forms tubers (Fig. 9B, C) similar to Orchidioides. The two sections have very similar trap and calyx morphologies (Taylor, 1989).

Orchid-like Utricularia species. Sect. Orchidioides: (A) U. quelchii N.E.Br.; (B) U. asplundii P.Taylor; (C) U. humboldtii Schomb.; (D) U. reniformis A.St.-Hil.; (E) U. nephrophylla Benj.; (F) U. alpina Jacq.; Sect. Iperua; (G) U. geminiloba Benj. Arrows denote the crest of the corolla. Photo credits: (A) Martin Hingst; (B) Nicole Rebbert (utricularien.de); (C) Barry Rice; (F) Ron Lane.
De Candolle (1844) created sect. Orchidioides, which included species with tubers. Kamieński (1895) later expanded this section and included non-tuberous species such as Utricularia nelumbifolia and Utricularia reniformis. Barnhart (1916), on the other hand, proposed the new genus Orchyllium to aggregate the species, with U. alpina (as Orchyllium alpinum) as the type species. Huynh (1968) questioned sect. Orchidioides, since it included species of different groups based on pollen characters. Taylor (1986), based on the morphological differences of the corolla, seeds and pollen, split sect. Orchidioides and proposed sect. Iperua, with Utricularia humboldtii Schomb. as the type species.
There are interesting discussions concerning the generic morphology of Utricularia and whether or not the species have a clear bauplan and delimitated organs as in other angiosperms or whether they fit within the Fuzzy Arberian Morphology (FAM) concept (Rutishauser and Isler, 2001; Rutishauser, 2016). Thus, it is not an easy task to classify species by means of morphological characters. Taylor (1989), in his monograph, raises some doubts about Utricularia morphology. In the Orchidioides and Iperua sections specifically, U. reniformis was questioned as it has thick, tuber-like stolons.
Recent molecular studies have also considered these sections using a phylogenetic approach. Jobson et al. (2003) show the monophyly of the Iperua and Orchidioides sections with the plastid sequences rps16 and trnL-F; Müller and Borsch (2005), on the other hand, proposed the exclusion of sect. Iperua on the basis of the plastid intron trnK with the matK gene.
Thus, our aim was to test the hypothesis of grouping the sections Orchidioides and Iperua by studying a broad range of species; we evaluated 11 of the 15 that have been described. A large number of molecular markers from two genomic compartments [plastid DNA sequences rps16, trnL-F and matK and nuclear internal transcribed spacer (ITS) region] and relevant morphological characters were evaluated. We also included the recently described Utricularia cornigera Studnička, a species with a morphological similarity to sect. Iperua (Studnička, 2009). We also conducted a phylogenetic analysis of the following morphological characteristics: (1) the crest on the lower lip of the corolla; (2) primary organs in the embryo; and (3) the presence of tubers. We conducted a histochemical analysis, with a discussion of the function and evolution of these organs.
MATERIALS AND METHODS
Plant samples and DNA markers
Plant samples from 20 species were obtained from both natural populations and cultivated plants and DNA sequences were also obtained from GenBank/NCBI (Table 1). In accordance with previous studies, four DNA sequences were selected as markers due to their phylogenetic signal: (1) rps16 (Oxelman et al., 1997; Jobson and Albert 2002; Jobson et al., 2003); (2) trnL-F (Taberlet et al., 1991; Jobson and Albert 2002; Jobson et al., 2003); (3) matK (Müller and Borsch, 2005; Silva et al., 2016); and (4) the ITS region (Hillis and Dixon, 1991). We therefore obtained a total of 76 sequences, of which 26 were produced in this study, with sequences for five species from Utricularia sect. Orchidioides (which has a total of nine described species) and six for sect. Iperua, including Utricularia cornigera, thus representing all known species of this section. In addition, 14 species from other sections were used as an external group (Table 1).
Utricularia species included in this study, their origin and GenBank access numbers, by molecular marker
| Section . | Species (voucher) . | GenBank access number . | |||
|---|---|---|---|---|---|
| rps16 . | trnL-F . | matK . | ITS . | ||
| Orchidioides | U. asplundii1 (TS000261) | AF482558·1 | AF482631·1 | KY68970 | KY689711 |
| U. quelchii1 (TS000260) | – | KY689702 | AF531846·1 | – | |
| U. endresii1 (TS000262) | – | AF482642·1 | KY799062 | KY689709 | |
| U. alpina1 (TS000263) | AF482556·1 | AF482629·1 | AF531822·1 | KY689712 | |
| U. praetermissa1 (TS000264) | – | KY689703 | KY689698 | KY689705 | |
| Iperua | U. humboldtii2 (TS000199) | – | KY689704 | AF531836·1 | – |
| U. geminiloba1 (VFOM2045) | – | AF482646·1 | KX604216·1 | KY689716 | |
| U. nephrophylla1 (VFOM2047) | AF482588·1 | AF482664·1 | AF531827·1 | KY689707 | |
| U. reniformis (VFOM2044) | AF482595·1 | AF482671·1 | KX604218·1 | KY689706 | |
| U. nelumbifolia1 (VFOM2055) | AF482586·1 | AF482662·1 | KX604217·1 | KY689708 | |
| U. cornigera1 (TS000265) | – | KY689701 | KY689699 | KY689710 | |
| Utricularia | U. aurea3 (TS000267) | AF482559·1 | AF482632·1 | KX604176·1 | KY689714 |
| U. australis | AF482560·1 | AF482633·1 | AF531823·1 | – | |
| U. intermedia | AF482575·1 | AF482651·1 | AF531839·1 | – | |
| U. macrorhiza3 (TS000266) | AF482581·1 | AF482657·1 | AF531835·1 | KY689719 | |
| U. minor3 (TS000268) | – | GU169706·1 | JN894028·1 | KY689721 | |
| U. vulgaris3 (TS000269) | – | JQ728994·1 | JN894054·1 | KY689722 | |
| Psyllosperma | U. huntii | AF482574·1 | AF482650·1 | – | – |
| U. praelonga | AF482591·1 | AF482667·1 | AF531843·1 | – | |
| U. longifolia1 (VFOM1680) | AF482580·1 | AF482656·1 | AF531834·1 | KY689718 | |
| U. hispida1 (VFOM1637) | – | – | AF531829·1 | KY689717 | |
| U. calycifida | – | – | AF531824·1 | – | |
| Foliosa | U. tricolor1 (VFOM2043) | AF482600·1 | AF482677·1 | KX604210·1 | KY689720 |
| U. tridentata | – | – | AF531825·1 | – | |
| U. amethystina1 (VFOM1644) | AF482557·1 | AF482630·1 | – | KY689713 | |
| Section . | Species (voucher) . | GenBank access number . | |||
|---|---|---|---|---|---|
| rps16 . | trnL-F . | matK . | ITS . | ||
| Orchidioides | U. asplundii1 (TS000261) | AF482558·1 | AF482631·1 | KY68970 | KY689711 |
| U. quelchii1 (TS000260) | – | KY689702 | AF531846·1 | – | |
| U. endresii1 (TS000262) | – | AF482642·1 | KY799062 | KY689709 | |
| U. alpina1 (TS000263) | AF482556·1 | AF482629·1 | AF531822·1 | KY689712 | |
| U. praetermissa1 (TS000264) | – | KY689703 | KY689698 | KY689705 | |
| Iperua | U. humboldtii2 (TS000199) | – | KY689704 | AF531836·1 | – |
| U. geminiloba1 (VFOM2045) | – | AF482646·1 | KX604216·1 | KY689716 | |
| U. nephrophylla1 (VFOM2047) | AF482588·1 | AF482664·1 | AF531827·1 | KY689707 | |
| U. reniformis (VFOM2044) | AF482595·1 | AF482671·1 | KX604218·1 | KY689706 | |
| U. nelumbifolia1 (VFOM2055) | AF482586·1 | AF482662·1 | KX604217·1 | KY689708 | |
| U. cornigera1 (TS000265) | – | KY689701 | KY689699 | KY689710 | |
| Utricularia | U. aurea3 (TS000267) | AF482559·1 | AF482632·1 | KX604176·1 | KY689714 |
| U. australis | AF482560·1 | AF482633·1 | AF531823·1 | – | |
| U. intermedia | AF482575·1 | AF482651·1 | AF531839·1 | – | |
| U. macrorhiza3 (TS000266) | AF482581·1 | AF482657·1 | AF531835·1 | KY689719 | |
| U. minor3 (TS000268) | – | GU169706·1 | JN894028·1 | KY689721 | |
| U. vulgaris3 (TS000269) | – | JQ728994·1 | JN894054·1 | KY689722 | |
| Psyllosperma | U. huntii | AF482574·1 | AF482650·1 | – | – |
| U. praelonga | AF482591·1 | AF482667·1 | AF531843·1 | – | |
| U. longifolia1 (VFOM1680) | AF482580·1 | AF482656·1 | AF531834·1 | KY689718 | |
| U. hispida1 (VFOM1637) | – | – | AF531829·1 | KY689717 | |
| U. calycifida | – | – | AF531824·1 | – | |
| Foliosa | U. tricolor1 (VFOM2043) | AF482600·1 | AF482677·1 | KX604210·1 | KY689720 |
| U. tridentata | – | – | AF531825·1 | – | |
| U. amethystina1 (VFOM1644) | AF482557·1 | AF482630·1 | – | KY689713 | |
Samples sequenced in this study: 1Carnivorous Plants Collection – Carlos Rohrbacher;
Carnivorous Plant Collection – Barry Rice;
Carnivorous Plant Collection – Institute of Botany of the Czech Academy of Sciences, Třeboň, Czech Republic (vouchers deposited in Herbarium JABU – University of Sao Paulo State – UNESP/FCAV).
Utricularia species included in this study, their origin and GenBank access numbers, by molecular marker
| Section . | Species (voucher) . | GenBank access number . | |||
|---|---|---|---|---|---|
| rps16 . | trnL-F . | matK . | ITS . | ||
| Orchidioides | U. asplundii1 (TS000261) | AF482558·1 | AF482631·1 | KY68970 | KY689711 |
| U. quelchii1 (TS000260) | – | KY689702 | AF531846·1 | – | |
| U. endresii1 (TS000262) | – | AF482642·1 | KY799062 | KY689709 | |
| U. alpina1 (TS000263) | AF482556·1 | AF482629·1 | AF531822·1 | KY689712 | |
| U. praetermissa1 (TS000264) | – | KY689703 | KY689698 | KY689705 | |
| Iperua | U. humboldtii2 (TS000199) | – | KY689704 | AF531836·1 | – |
| U. geminiloba1 (VFOM2045) | – | AF482646·1 | KX604216·1 | KY689716 | |
| U. nephrophylla1 (VFOM2047) | AF482588·1 | AF482664·1 | AF531827·1 | KY689707 | |
| U. reniformis (VFOM2044) | AF482595·1 | AF482671·1 | KX604218·1 | KY689706 | |
| U. nelumbifolia1 (VFOM2055) | AF482586·1 | AF482662·1 | KX604217·1 | KY689708 | |
| U. cornigera1 (TS000265) | – | KY689701 | KY689699 | KY689710 | |
| Utricularia | U. aurea3 (TS000267) | AF482559·1 | AF482632·1 | KX604176·1 | KY689714 |
| U. australis | AF482560·1 | AF482633·1 | AF531823·1 | – | |
| U. intermedia | AF482575·1 | AF482651·1 | AF531839·1 | – | |
| U. macrorhiza3 (TS000266) | AF482581·1 | AF482657·1 | AF531835·1 | KY689719 | |
| U. minor3 (TS000268) | – | GU169706·1 | JN894028·1 | KY689721 | |
| U. vulgaris3 (TS000269) | – | JQ728994·1 | JN894054·1 | KY689722 | |
| Psyllosperma | U. huntii | AF482574·1 | AF482650·1 | – | – |
| U. praelonga | AF482591·1 | AF482667·1 | AF531843·1 | – | |
| U. longifolia1 (VFOM1680) | AF482580·1 | AF482656·1 | AF531834·1 | KY689718 | |
| U. hispida1 (VFOM1637) | – | – | AF531829·1 | KY689717 | |
| U. calycifida | – | – | AF531824·1 | – | |
| Foliosa | U. tricolor1 (VFOM2043) | AF482600·1 | AF482677·1 | KX604210·1 | KY689720 |
| U. tridentata | – | – | AF531825·1 | – | |
| U. amethystina1 (VFOM1644) | AF482557·1 | AF482630·1 | – | KY689713 | |
| Section . | Species (voucher) . | GenBank access number . | |||
|---|---|---|---|---|---|
| rps16 . | trnL-F . | matK . | ITS . | ||
| Orchidioides | U. asplundii1 (TS000261) | AF482558·1 | AF482631·1 | KY68970 | KY689711 |
| U. quelchii1 (TS000260) | – | KY689702 | AF531846·1 | – | |
| U. endresii1 (TS000262) | – | AF482642·1 | KY799062 | KY689709 | |
| U. alpina1 (TS000263) | AF482556·1 | AF482629·1 | AF531822·1 | KY689712 | |
| U. praetermissa1 (TS000264) | – | KY689703 | KY689698 | KY689705 | |
| Iperua | U. humboldtii2 (TS000199) | – | KY689704 | AF531836·1 | – |
| U. geminiloba1 (VFOM2045) | – | AF482646·1 | KX604216·1 | KY689716 | |
| U. nephrophylla1 (VFOM2047) | AF482588·1 | AF482664·1 | AF531827·1 | KY689707 | |
| U. reniformis (VFOM2044) | AF482595·1 | AF482671·1 | KX604218·1 | KY689706 | |
| U. nelumbifolia1 (VFOM2055) | AF482586·1 | AF482662·1 | KX604217·1 | KY689708 | |
| U. cornigera1 (TS000265) | – | KY689701 | KY689699 | KY689710 | |
| Utricularia | U. aurea3 (TS000267) | AF482559·1 | AF482632·1 | KX604176·1 | KY689714 |
| U. australis | AF482560·1 | AF482633·1 | AF531823·1 | – | |
| U. intermedia | AF482575·1 | AF482651·1 | AF531839·1 | – | |
| U. macrorhiza3 (TS000266) | AF482581·1 | AF482657·1 | AF531835·1 | KY689719 | |
| U. minor3 (TS000268) | – | GU169706·1 | JN894028·1 | KY689721 | |
| U. vulgaris3 (TS000269) | – | JQ728994·1 | JN894054·1 | KY689722 | |
| Psyllosperma | U. huntii | AF482574·1 | AF482650·1 | – | – |
| U. praelonga | AF482591·1 | AF482667·1 | AF531843·1 | – | |
| U. longifolia1 (VFOM1680) | AF482580·1 | AF482656·1 | AF531834·1 | KY689718 | |
| U. hispida1 (VFOM1637) | – | – | AF531829·1 | KY689717 | |
| U. calycifida | – | – | AF531824·1 | – | |
| Foliosa | U. tricolor1 (VFOM2043) | AF482600·1 | AF482677·1 | KX604210·1 | KY689720 |
| U. tridentata | – | – | AF531825·1 | – | |
| U. amethystina1 (VFOM1644) | AF482557·1 | AF482630·1 | – | KY689713 | |
Samples sequenced in this study: 1Carnivorous Plants Collection – Carlos Rohrbacher;
Carnivorous Plant Collection – Barry Rice;
Carnivorous Plant Collection – Institute of Botany of the Czech Academy of Sciences, Třeboň, Czech Republic (vouchers deposited in Herbarium JABU – University of Sao Paulo State – UNESP/FCAV).
Amplification and sequencing
The DNA was extracted by the CTAB method (Doyle and Doyle, 1987), modified by Lodhi et al. (1994). Amplification reactions of the nuclear markers were conducted in 25 μL of a solution containing 20 mm MgCl2, 100 mm dNTPs, 10 mm of each primer, 1 U of Dream Taq Polymerase (Fermentas), and on average 50 ng of DNA template. For the ITS region, the primers and dimethyl sulphoxide adjuvant (DMSO) were used as recommended by Miranda et al. (2010). For matK and trnL-F the primers employed were according Lim et al. (2012) and Taberlet et al. (1991), respectively.
The thermal profile of the amplification reactions for the intergenic spacer trnL-F was 95 °C for 3 min; 30 cycles of 1 min at 94 °C, 45 s at 52 °C and 1 min at 72 °C, and 5 min of final extension at 72 °C. For the gene matK, it was 94 °C for 1 min, 35 cycles of 40 s at 94 °C, 20 s at 52 °C and 50 s at 72 °C, and 10 min of final extension at 72 °C. For the nuclear ITS, it was 95 °C for 3 min, 35 cycles of 30 s at 95 °C, 30 s at 54 °C and 1 min at 72 °C, and 5 min of final extension at 72 °C.
The amplicons were verified by 0·8 % agarose gel electrophoresis, precipitated with 100 % isopropanol, purified with 70 % ethanol and sequenced by the method developed by Sanger et al. (1975) in an automatic sequencer, model 3730xl ABI (Applied Biosystems).
Sequences and phylogenetic analyses
The identity of each sequence was determined with the BLASTN application (Altschul et al., 1990). Using Geneious v10.0 (Kearse et al., 2012), their Phred quality was verified and visual and manual adjustments were made with the program BioEdit v7.5.0.2 (Hall, 1999). MAFFT v7 (Katoh et al., 2002) was used to align the sequences, and datasets (matrices) were created with BioEdit v7.5.0.2 (Hall, 1999) with the addition of masks (missing data = ‘?’) (Wiens, 2006).
We produced five datasets: four for each isolated marker and one for a combined (total evidence) analysis. For the matK fragment, the sequences obtained from GenBank (Table 1) were trimmed to achieve a homologue region according to the amplified sequences by the 3F_KIM and 1R_KIM primers (Lim et al., 2012).
In each dataset generated, a Bayesian inference on platform CIPRES (Miller et al., 2010) was performed, in which the best fit was employed, as selected by the Akaike information criterion (AIC) (Akaike, 1973), generated by the program jModelTest v2.1.1 (Darriba et al., 2012). The best-fit model for the plastid markers was the GTR + G model (Tavaré, 1986) and that for the nuclear spacer was the TIM3 + I + G model. Additionally, we performed maximum likelihood (ML) analysis with the CIPRES platform (Miller et al., 2010) and the PAUP*4.0 program (Swofford, 2003) to get bootstrap values (2000 pseudoreplicates and heuristic search with 1000 replicates with random addition of sequences and the branch swapping algorithm TBR). The trees obtained were edited with TreeGraph 2 (Stöver, 2010) and FigTree v1.3.1 (Rambaut, 2009).
Morphological characters
We conducted phylogenetic testing of the three morphological characteristics that supposedly emphasize the differences between the sections and have therefore been employed historically in the taxonomic circumscription of Orchidioides and Iperua. These were: (1) a crest on the protuberance of the lower lip of the corolla (Fig. 1C, E); (2) the presence of tubers (Taylor, 1986, 1989); and (3) the primary organs in the embryo, according to Płachno and Świątek (2010). A morphological matrix was generated using the NDE Nexus Data Editor program (Page, 2001), which overlapped the combined (total evidence) Bayesian inference tree, for which the Mesquite program (Maddison and Maddison, 2010) was used.
Histochemistry of storage organs and tissues
To achieve better comprehension and comparison of storage tissues, we conducted histochemical analyses of the stolons of Utricularia reniformis and Utricularia nelumbifolia (sect. Iperua) and the tubers of Utricularia geminiloba (sect. Iperua) and Utricularia alpina (sect. Orchidioides). The stolons of U. reniformis and U. nelumbifolia and tubers of U. geminiloba were collected from natural populations in December 2015 or were taken from a collection in the Botanic Gardens of Jagiellonian University in Kraków, Poland, and the vouchers were deposited in Herbarium JABU (V.F.O. de Miranda et al., 2044, 2055 and 2045, respectively).
The following reagents were used to show the general anatomy and the storage components: IKI (iodine–potassium iodide) for starch + proteins; saturated ethanolic solutions of Sudan III and Sudan IV for lipids; alum carmine and iodine green for lignin + cellulose; and 0·1 % (w/v) ruthenium red for pectin and mucilage (Filutowicz and Kużdowicz 1951; Ruzin 1999).
The material (thick stolons of U. reniformis and U. nelumbifolia and tubers of U. geminiloba and U. alpina) was fixed in 2·5 % (v/v) glutaraldehyde/2·5 % (v/v) formaldehyde in 0·05 m sodium cacodylate buffer (pH 7·0) for several days, washed three times in the same buffer and postfixed in 1% (w/v) osmium tetroxide solution for 1·5 h at 0 °C. This was followed by dehydration using a graded ethanol series and infiltration and embedding using an epoxy embedding medium kit (Fluka).
Semithin sections (0·9–1·0 µm) prepared for light microscopy were stained for general histology using aqueous methylene blue/azure II (MB/AII) for 1–2 min (Humphrey and Pittman, 1974) and examined with an Olympus BX60 optical microscope.
Photosynthetic function of stolons and tubers
To verify the presence of chloroplasts and possible photosynthetic activity in stolons and tubers, these organs were used in a glasshouse experiment. Three stolon fragments (∼2 cm) of U. reniformis and three tubers of U. geminiloba were placed in Petri dishes with moist absorbent paper and stored at 25 °C under natural light conditions for 30 d. Photographs of the fragments and tubers were then taken.
RESULTS
Phylogeny of Utricularia sections Orchidioides and Iperua
The phylogenetic trees from the Bayesian inference and ML criteria are congruent in their general topology (Figs 2 and 3) and showed that most groups were supported by posterior probabilities (PPs) and ML bootstraps (> = 50%) (Table 2).
Matrices and statistical analyses of alignments and cladograms inferred by maximum likelihood (ML) and Bayesian inference (BI)
| Dataset . | Genome . | Terminals, n . | Characters considering gaps, bp . | Clades with support ≥50 %, n (%)1 . | |
|---|---|---|---|---|---|
| Posterior probability (BI) . | Bootstraps (ML) . | ||||
| rps16 | Plastid | 14 | 926 | 11 (84) | 10 (77) |
| trnL-trnF | Plastid | 22 | 1·091 | 16 (76) | 12 (57) |
| matK2 | Plastid | 23 | 883 | 19 (86) | 16 (72) |
| ITS | Nucleus | 17 | 962 | 12 (75) | 10 (62) |
| Combined | Nucleus + Plastid | 25 | 3·864 | 20 (83) | 17 (71) |
| Dataset . | Genome . | Terminals, n . | Characters considering gaps, bp . | Clades with support ≥50 %, n (%)1 . | |
|---|---|---|---|---|---|
| Posterior probability (BI) . | Bootstraps (ML) . | ||||
| rps16 | Plastid | 14 | 926 | 11 (84) | 10 (77) |
| trnL-trnF | Plastid | 22 | 1·091 | 16 (76) | 12 (57) |
| matK2 | Plastid | 23 | 883 | 19 (86) | 16 (72) |
| ITS | Nucleus | 17 | 962 | 12 (75) | 10 (62) |
| Combined | Nucleus + Plastid | 25 | 3·864 | 20 (83) | 17 (71) |
Percentage of clades was calculated from the total of possible tree clades (= terminal numbers−1).
Sequences obtained from GenBank (see Table 1) were trimmed to achieve a homologous region according to the fragment amplified by the 3F_KIM and 1R_KIM primers (Lim et al., 2012).
Matrices and statistical analyses of alignments and cladograms inferred by maximum likelihood (ML) and Bayesian inference (BI)
| Dataset . | Genome . | Terminals, n . | Characters considering gaps, bp . | Clades with support ≥50 %, n (%)1 . | |
|---|---|---|---|---|---|
| Posterior probability (BI) . | Bootstraps (ML) . | ||||
| rps16 | Plastid | 14 | 926 | 11 (84) | 10 (77) |
| trnL-trnF | Plastid | 22 | 1·091 | 16 (76) | 12 (57) |
| matK2 | Plastid | 23 | 883 | 19 (86) | 16 (72) |
| ITS | Nucleus | 17 | 962 | 12 (75) | 10 (62) |
| Combined | Nucleus + Plastid | 25 | 3·864 | 20 (83) | 17 (71) |
| Dataset . | Genome . | Terminals, n . | Characters considering gaps, bp . | Clades with support ≥50 %, n (%)1 . | |
|---|---|---|---|---|---|
| Posterior probability (BI) . | Bootstraps (ML) . | ||||
| rps16 | Plastid | 14 | 926 | 11 (84) | 10 (77) |
| trnL-trnF | Plastid | 22 | 1·091 | 16 (76) | 12 (57) |
| matK2 | Plastid | 23 | 883 | 19 (86) | 16 (72) |
| ITS | Nucleus | 17 | 962 | 12 (75) | 10 (62) |
| Combined | Nucleus + Plastid | 25 | 3·864 | 20 (83) | 17 (71) |
Percentage of clades was calculated from the total of possible tree clades (= terminal numbers−1).
Sequences obtained from GenBank (see Table 1) were trimmed to achieve a homologous region according to the fragment amplified by the 3F_KIM and 1R_KIM primers (Lim et al., 2012).
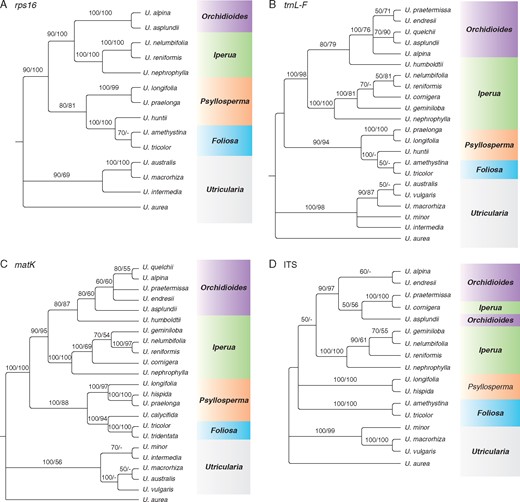
Bayesian inference trees for (A) rps16, (B) trnL-F, (C) matK and (D) ITS. Numbers above the branches are posterior probabilities followed by maximum likelihood bootstraps. −, branches with support value <50.
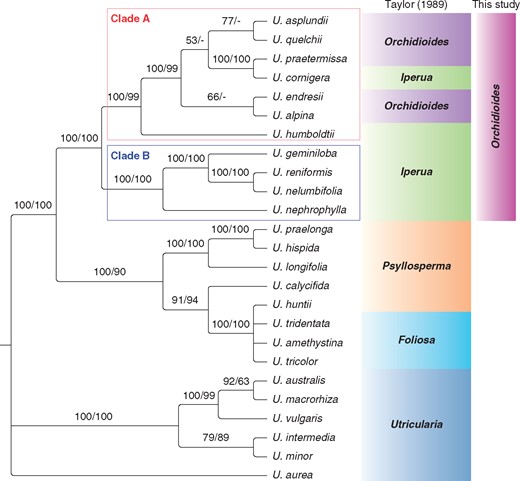
Bayesian inference for the combined analysis (rps16 + trnL-F + matK + ITS). Numbers above the branches are the posterior probabilities followed by maximum likelihood bootstraps. −, branches with support value <50.
In the analysis of intron rps16, in which the sequences of the studied sections taken from the study of Jobson and Albert (2002) were used, a similar result, congruent to Taylor’s classification (1989), was found (Fig. 2A). The other plastid markers (trnL-F and matK; Fig. 2B, C) show sect. Iperua as a paraphyletic group, by the inclusion of U. humboldtii (taxonomically recognized as belonging to sect. Iperua) in sect. Orchidioides. Furthermore, the sequences trnL-F and matK placed U. cornigera in sect. Iperua. The tree obtained with the spacer trnL-F (Fig. 2B) revealed that U. humboldtii is a sister group to the other species in sect. Orchidioides and that U. cornigera should be included in sect. Iperua as a sister group to the clade U. nelumbifolia–U. reniformis. With this marker, the ML analysis showed the species U. nelumbifolia, U. reniformis, U. cornigera and U. geminiloba as a monophyletic group (Fig. 2B). U. humboldtii has a similar position, being nested as an external branch of the clade formed by U. praetermissa, U. endresii, U. quelchii, U. asplundii and U. alpina. The matK tree (Fig. 2C) revealed that U. humboldtii is closely related to sect. Orchidioides as a sister group, because a similar topology to the trnL-F dataset was found (Fig. 2B).
Additionally, while the plastid markers trnL-F and matK showed U. cornigera nested in the sect. Iperua clade, the ITS dataset (Fig. 2D) showed this species grouped in sect. Orchidioides as a sister group of U. praetermissa, with maximum support values from both analyses (100 % for PP and ML bootstrap). Sections Iperua and Orchidioides were shown as paraphyletic (Fig. 2D), and U. humboldtii is missing in this analysis.
The tree resulting from the concatenated datasets presents sect. Iperua as a paraphyletic group, which shows U. humboldtii as an external branch of sect. Orchidioides (Fig. 3, clade A). Utricularia cornigera is shown in the clade of sect. Orchidioides, as a monophyletic group with U. asplundii, U. quelchii and U. praetermissa, but this clade is not strongly supported (PP 53 % and ML bootstrap <50 %). Despite this low support, the clade of sect. Orchidioides, which includes U. cornigera, is strongly supported by the Bayesian inference and ML bootstrap, with 100 and 99 % confidence, respectively (Fig. 3).
Distribution of morphological characters
The characteristics of both the crests on the corolla lower lip (Fig. 4A) and the tubers (Fig. 4C) do no support as synapomorphies to the sections Orchidioides and Iperua. According to the combined analysis, the crest on the corolla has possibly appeared at least twice. It was once present in the ancestral lineage of Orchidioides–Iperua complex (clade A + clade B), which was lost by an ancestor of the clade Iperua only to arise again as a reversion in U. cornigera. The character of the tubers was also shown to be homoplastic, with two independent origins: one in sect. Orchidioides, with the reversion to U. cornigera, which lacks tubers, and the other in U. geminiloba. The characteristic primary embryo organs (Fig. 4B) occur in U. cornigera (Studnička, 2009), U. humboldtii, U. nelumbifolia and species of sect. Utricularia (Płachno and Świątek, 2010 and references therein). The lack of information regarding the embryology of several species makes it difficult to trace a robust and well-supported hypothesis for the evolution of this character.
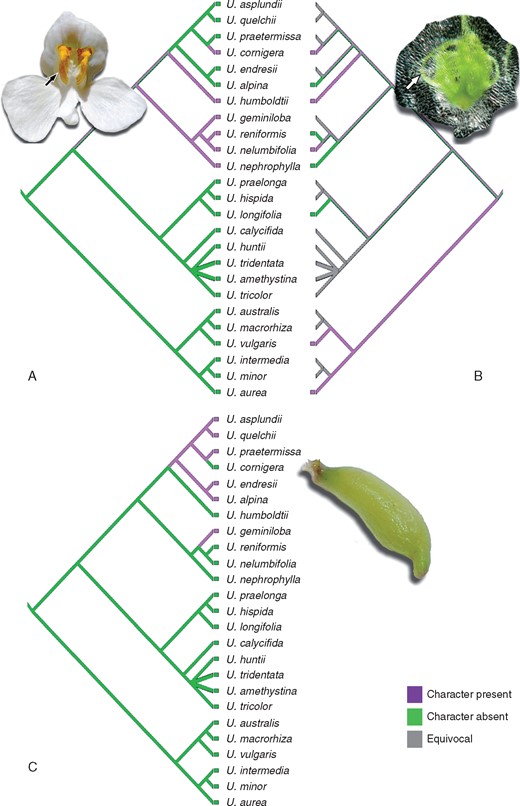
Distribution of morphological characters based on the Bayesian combined tree. (A) Crest on the lower lip of the corolla. Primary organs in the embryo (B) and tubers (C). Photo credit: Barry Rice, detail in (B).
Histological analysis of stolons and tubers
In our analyses, the thick stolons of U reniformis (Figs 5 and 9A) and U. nelumbifolia (Fig. 7) and the tubers of U. geminiloba (Figs 6 and9B, C) and U. alpina (Fig. 8) have similar anatomy. In general, as shown in the transverse sections, the epidermis and parenchymatous cortex surround the ectophloic central cylinder. Only the stolons of U. nelumbifolia have many lacunae (Fig. 7A–D). The cortex consists of large parenchyma cells with large vacuoles; the xylem and phloem elements are separated from each other. In both organs small epidermal trichomes occur, each consisting of one basal cell, one short, central cell and a long-headed cell (Fig. 7E).
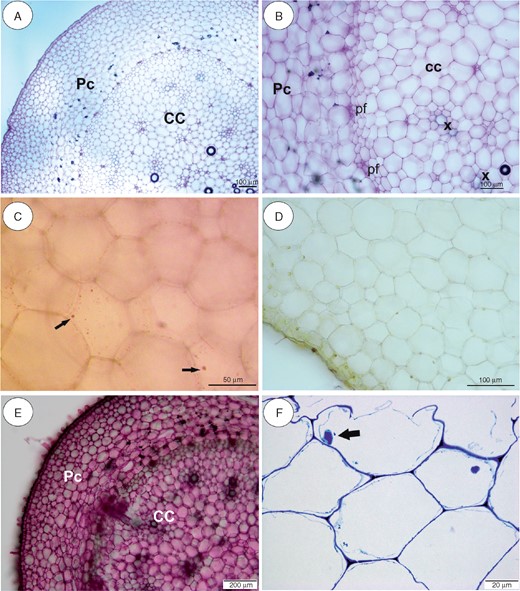
Anatomy and histochemistry of Utricularia reniformis stolons. (A, B) General stolon anatomy. Note the parenchymatous cortex (Pc), which surrounds the ectophloic central cylinder (CC). pf, phloem; x, xylem. Scale bar = 100 µm. (C) Reaction for lipids (Sudan IV). Arrows indicate small lipid droplets. Scale bar = 50 µm. (D) Section after IKI treatment. Note the lack of starch grains. Scale bar = 100 µm. (E) Section treated with ruthenium red for pectins and mucilage. Note the positive pectin reaction in cell walls. Scale bar = 200 µm. (F) Semithin section. Note the giant vacuoles and the nucleus with paracrystalline protein inclusions. Scale bar = 20 µm.
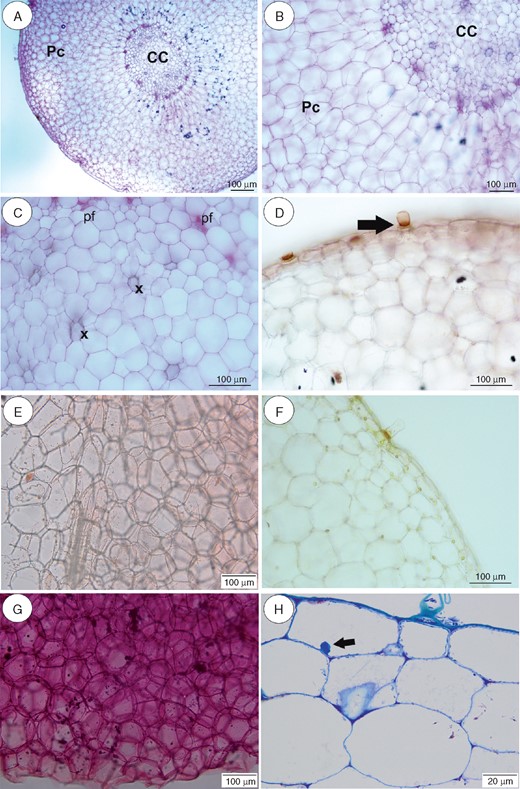
Anatomy and histochemistry of Utricularia geminiloba tubers. (A–C) General tuber anatomy. Note the parenchymatous cortex (Pc), which surrounds the ectophloic central cylinder (CC). pf, phloem; x, xylem. Scale bar = 100 µm. (D) Reaction for lipids (Sudan IV). Note positive reaction in barrier cell of the trichome (arrow). Scale bar = 100 µm. (E) Reaction for lipids (Sudan IV). Scale bar = 100 µm. (F) Section after IKI treatment. Note the lack of starch grains, but the positive reaction for protein in the nuclei and trichome. Scale bar = 100 µm. (G) Section treated with ruthenium red for pectins and mucilage. Note (arrow) the positive pectin reaction in cell walls. Scale bar = 100 µm. (H) Semithin section. Note the giant vacuoles and the nucleus with paracrystalline protein inclusion. Scale bar = 20 µm.
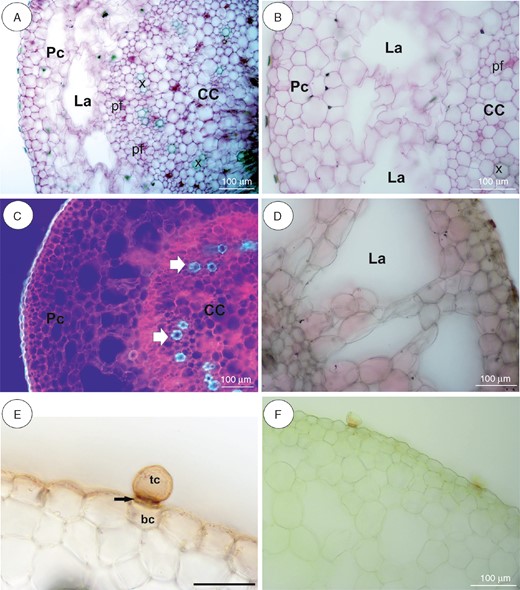
Anatomy and histochemistry of Utricularia nelumbifolia stolons. (A, B) General stolon anatomy. Note the parenchymatous cortex (Pc), which surrounds the ectophloic central cylinder (CC). pf, phloem; x, xylem; La, lacunae. Scale bar = 100 µm. (C) Autofluorescence of tissues under UV light. Pc, parenchymatous cortex; CC, central cylinder (CC). xylem. Arrow indicates xylem. Scale bar = 100 µm. (D) Negative reaction for lipids (Sudan III). Scale bar = 100 µm. (E) Reaction for lipids (Sudan IV). Note the positive reaction in cuticle of epidermal cells and the barrier cell of the trichome. tc, terminal cell; bc, basal cell. Arrow indicates a barrier cell. Scale bar = 50 µm. (F) Section after IKI treatment. Note the lack of starch grains. Scale bar = 100 µm.
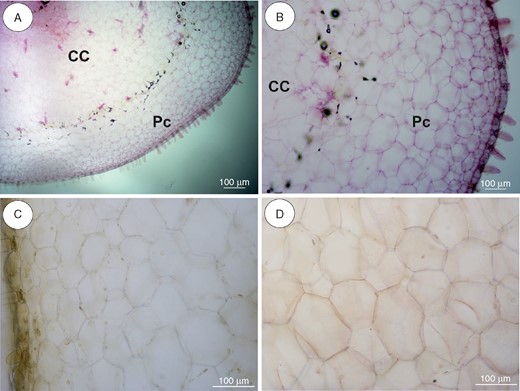
Anatomy and histochemistry of Utricularia alpina tubers. (A, B) General tuber anatomy. Note the parenchymatous cortex (Pc), which surrounds the ectophloic central cylinder (CC). Scale bar = 100 µm. (C) Section after IKI treatment, showing positive reaction for protein in nuclei. Scale bar = 100 µm. (D) Reaction for lipids (Sudan IV). Scale bar = 100 µm.
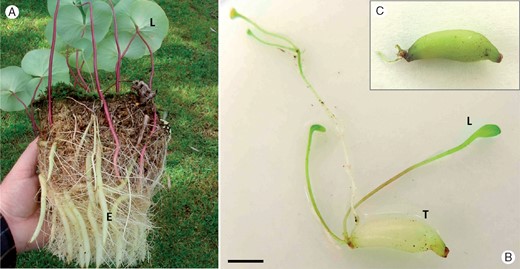
Habit of Utricularia reniformis (A) and tuber of Utricularia geminiloba before (B) and after (C) a 30-d experiment to verify the photosynthetic function. L, leaf; E, stolon; T, tuber. Photo credit for (A): David Banks. Scale bar = 5 mm.
Very small and infrequent lipid droplets were observed in the cytoplasm of the cortical cells (Fig. 5C). Cell walls stained with the ruthenium red (Figs 5E and 6G) showed no mucilage and those stained with IKI showed no starch or storage proteins (Figs 5D, 6F, 7F and 8C). Paracrystalline protein inclusions were occasionally present in the nuclei of various cell types (Figs 5F and 6H). The cell walls of the trichome barrier cell stained selectively with Sudan (Figs 6D and 7E).
DISCUSSION
Phylogeny of Orchidioides–Iperua complex: one section is enough
Our results support the paraphyly of Utricularia sect. Iperua and Orchidioides, considering U. cornigera (Figs 2 and 3). The key species that makes sect. Iperua paraphyletic is U. humboldtii, which is the type species assigned to this section (Taylor, 1986, 1989). Similar results were obtained by Müller et al. (2004) based on matK and the flanking trnK intron, and later Müller and Borsch (2005) suggested acceptance of only sect. Orchidioides, underpinning De Candolle (1844) and Kamieński (1895). De Candolle based his system on only three species of the complex: U. alpina (as U. montana) and U. unifolia, classified as sect. Orchidioides and, curiously, U. humboldtii, which was maintained in his ‘Species dubiae’ section.
Utricularia humboldtii (Fig. 1C), a perennial and one of the largest terrestrial species of the genus, produces spectacular flowers, which is a common trait of the Orchidioides–Iperua complex. This species is found as a terrestrial or even as an aquatic epiphyte, since the plants can project aerial horizontal stolons that reach and grow between bromeliad leaves (Taylor, 1989). While the species of sect. Iperua are distributed in South America, mainly in Southern and Southeastern Brazil (BFG, 2015; Miranda et al., 2015), U. humboldtii is the only species of this section that occurs in the Guiana Highlands. Thus, clades A and B (Fig. 3) are also supported by species distribution (with the exception of U. cornigera, which is endemic to Southeastern Brazil) (BFG, 2015; Miranda et al., 2015).
Utricularia cornigera Studnička
In our analysis with the plastid markers trnL-F (Fig. 2B) and matK (Fig. 2C), U. cornigera is nested into sect. Iperua and related to the clade U. reniformis–U. nelumbifolia and also nested with U. geminiloba, despite the incongruence found with this species when considering both markers. The hypothesis of a natural hybrid, originating from the crossing between U. reniformis and U. nelumbifolia (Fleischmann, 2012), has been refuted by Studnička (2013, 2015) by an interbreeding experiment.
However, in the ITS analysis U. cornigera is included in sect. Orchidioides, with maximum statistical support in both phylogenetic analyses (PP and ML), and it also remains in the Orchidioides in the concatenated tree (Fig. 3). While the maternal DNA (chloroplast DNA in this case) supports a close relationship with the species of sect. Iperua, the ITS region, which is of biparental origin, suggests that U. cornigera has the same common ancestral species related to the Orchidioides clade. The multiple copies of the ITS region found in eukaryotic genomes may be a problem when using these data for phylogenetic inferences, since it is not certain that the sequences achieved are orthologues (Miranda et al., 2010). Nonetheless, despite the multiple copies, concerted evolution occurs when sequence differences among copies in the same genome become homogenized to the same sequence by mechanisms such as high-frequency unequal crossing-over and gene conversion (Álvarez and Wendel, 2003). Considering that the importance of the ITS region for phylogenetic inferences is mainly due to its high signal, which can be used to solve the phylogeny of closely related taxa (Hillis and Dixon, 1991), the results presented in this study are very important. However, further analyses with the use of different nuclear markers should address this issue. The topologies presented by the ITS analysis and the combined analysis provide phylogenetic support for the recognition of U. cornigera as a species.
Distribution of morphological characters
Taylor (1986, 1989) used three main characters for justifying the splitting of sect. Orchidioides and for the creation of sect. Iperua: the crest on the lower lip of the corolla and the morphology of the seeds and pollen. The pollen has already been studied in the species of these sections by Huynh (1968), who placed U. alpina, U. praetermissa, U. jamesoniana and U. humboldtii into one group, similar to our molecular results, which included U. humboldtii in sect. Orchidioides (Fig. 3, clade A).
The characteristic crest on the lower lip of the corolla (Fig. 4A) is exclusive to sect. Iperua (Taylor, 1989). Since our molecular results showed that U. humboldtii is closely related to the clade of sect. Orchidioides (clade A), this character becomes worthless as a diagnostic feature for this section.
For the seeds and embryos, Taylor (1989) observed that those from sect. Orchidioides are more uniform, which is markedly different from the situation in sect. Iperua: in the latter, U. humboldtii and U. nelumbifolia form a thin and transparent testa, with chlorophyllose embryos having numerous primary organs (Goebel, 1891; Merl, 1915; Lloyd, 1942; and see arrow in Fig. 4B). The primary organs of the embryos of U. nelumbifolia and U. reniformis A.St.-Hil. ‘Enfant Terrible’ were studied by Płachno and Świątek (2010). In U. nelumbifolia, the primary organs are homologous to those found in the seedlings of sect. Utricularia; similar to the ‘leaf’ structure, but with the likely function of nutrient absorption from the environment. These numerous primary embryo organs are adaptations for germination in bromeliad tanks (Studnička, 2009, 2011). Płachno and Świątek (2010) also disagreed with sect. Iperua, as they verified that the embryos of U. nelumbifolia and U. reniformis sensu stricto had similar structures, unlike U. humboldtii. In the last species, the basal part of the embryo is not so prominent and the primary organs dominate (Goebel, 1893). In any case, the germination pattern of U. reniformis ‘Enfant Terrible’ is different from that of U. reniformis sensu stricto (Goebel, 1893; Merl, 1925), U. humboldtii and U. nelumbifolia. The embryo in U. nephrophylla does not have primordial ‘leaves’ (Merl, 1925) and/or, as in U. geminiloba, they are not well enough developed (Taylor, 1989) or are not seen (Płachno and Świątek, 2010). The newly described species U. cornigera (Studnička, 2009) has green embryos with numerous primary organs, unlike the ‘true’ U. reniformis.
Tubers occur as an important, specialized water storage organ in all species of sect. Orchidioides as these species are epiphytes (Taylor, 1989). In sect. Iperua tubers are only present in U. geminiloba, which is found as a lithophyte or a terrestrial. Despite the differences between these life forms, the epiphytic and lithophytic habitats may represent similar restrictions, with both having harsh and highly variable seasonal conditions. Utricularia geminiloba is commonly found on granitic walls with little organic matter (Taylor, 1989; V.F.O. Miranda, pers. observ.) and thus the tubers are an important water source in dry seasons.
Taylor (1989) observed great similarity between the tubers, and the stolons of U. reniformis (Fig. 7), which are thick, without constriction and almost like very elongated tubers. The author did not, however, obtain any further information about these structures.
Function and evolution of the stolon–tuber system: are there differences between stolons and tubers?
The anatomy of the thick stolons of U. reniformis and U. geminiloba tubers resembles the anatomy of the stolons of U. alpina (Brugger and Rutishauser, 1989) and U. longifolia (Rutishauser and Isler, 2001), but in this case with a more developed cortex. Unlike our observation of U. reniformis and U. geminiloba, the thick stolons of U. humboldtii (Brugger and Rutishauser, 1989) and U. nelumbifolia have many lacunae (Fig. 7). Our observation agrees with that of Adlassnig et al. (2005), in that the tubers of U. alpina are constituted of developed parenchyma cells forming a giant vacuole. Darwin (1875) also analysed the tubers of U. alpina and did not find starch, and suggested their function was water storage (Juniper et al., 1989).
Taylor (1989) recognized that the tubers in the species of sect. Orchidioides worked like orchid pseudobulbs, which ensures the species’ survival in dry periods. Due to this hydric stress and the species growing far above the soil, the acquisition and storage of water are the main abiotic factors for the growth of epiphytic species, whereas the availability of nutrients and solar irradiance remain secondary concerns (Zotz and Hietz, 2001; Laube and Zotz, 2003). Thus, in most epiphytes there are adaptive structures and pseudobulbs (Orchidaceae), velamen (Orchidaceae, Araceae, Bromeliaceae), and also leaf trichomes (Bromeliaceae), which facilitate the absorption of water (Benzing and Sheemann, 1978). Also, when exposed to light, the tubers and stolons can play an important role as photosynthetic organs (Fig. 9B, C).
In sect. Iperua, U. geminiloba is a terrestrial and lithophytic species that is often found on moist walls and can survive dry periods, like U. reniformis, which is terrestrial, epiphytic and also lithophytic. According to Taylor (1989), it is not always certain whether an Utricularia species is a holoepiphyte or a facultative or accidental epiphyte, as some species were rarely observed in their natural habitat. For example, U. alpina, although usually an epiphyte, occasionally grows on the ground (Taylor, 1989).
From this study it is possible to infer the close relationship between tubers and thick stolons as a crucial survival strategy for epiphytes and lithophytes. Despite this, the stolon–tuber system is linked with the species of sections Iperua and Orchidioides; other sections within the genus Utricularia also have species with tubers or tuber-like thick stolons (e.g. in sections Aranella, Chelidon, Phyllaria, Pleiochasia and Utricularia), and there is one species of Genlisea (Rivadavia et al., 2013). In these taxa there may also be a nutrient storage function, particularly for species found in poor and seasonally stressful environments. For example, U. menziesii has a dormancy period throughout the hot, dry Australian summer (Taylor, 1989). The tubers of this species may play an important role in carbohydrate storage (Rice, 2011), but further studies with histological analysis are necessary to prove this assumption. In this way, it is possible that the stolon–tuber system, with its primary function being water storage, originated independently (as homoplasies) within the genus Utricularia as an adaptation to the hydric deficits, with a common occurrence in the Orchidioides–Iperua complex.
Conclusions
Our phylogenetic analyses included U. humboldtii and U. cornigera in sect. Orchidioides and therefore do not support the separation of these sections made by Taylor (1986, 1989). In addition, the stolons and tubers of the species in sect. Iperua have anatomical patterns similar to those of the Orchidioides tubers and perform the same primary function as water storage organs, with a potentially common origin. Our results therefore support the joining of these sections into one section called Orchidioides, as suggested by Müller and Borsch (2005).
ACKNOWLEDGEMENTS
We dedicate our paper to Dr Miloslav Studnička (Liberec Botanic Garden, Czech Republic), who has been involved in studying Utricularia in the Orchidioides and Iperua sections for many years. Thanks are due to Drs Carlos Rohrbacher (Brazil) and Barry Rice (USA), who provided part of the plant material, and to Martin Hingst, Nicole Rebbert, Barry Rice, David Banks and Ron Lane for kindly providing the pictures of some species. Thanks to Drs Nilber Gonçalves da Silva and Yani Cristina Aranguren Díaz for helping during the field trips, and for fruitful discussions. Thanks are also due to horticulturist Lucyna Kurleto for her conscientious care of the collection of carnivorous plants in the Botanical Garden of Jagiellonian University in Kraków, Poland. Special thanks are due to Dr Brian G. McMillan (University of Strathclyde, Glasgow) for the correction of the language. This work was supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) (fellowships for F.G.R., N.F.M. and S.R.S.); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Bolsa de Produtividade, grant number 309040/2014-0 for V.F.O.M.); and partly (for L.A.) by the Research Programme of the Czech Academy of Sciences (RVO 67985939).



