-
PDF
- Split View
-
Views
-
Cite
Cite
Ana M. Sánchez, Patricia Alonso-Valiente, M. José Albert, Adrián Escudero, How might edaphic specialists in gypsum islands respond to climate change? Reciprocal sowing experiment to infer local adaptation and phenotypic plasticity, Annals of Botany, Volume 120, Issue 1, July 2017, Pages 135–146, https://doi.org/10.1093/aob/mcx046
Close - Share Icon Share
Abstract
Background and Aims Local adaptation and phenotypic plasticity are considered key mechanisms for coping with climate warming, especially for plant species that inhabit island-like habitats. In Spain a complete guild of edaphic specialists, most of them threatened, occurs in gypsum outcrops, but how these species will respond to climate change has received little attention.
Methods A reciprocal sowing experiment was performed to determine the extent of local adaptation and phenotypic plasticity in five gypsophytes with contrasting distributions along a climate gradient. Germination, seedling growth and survival were recorded during a 4-year period.
Key Results Plants responded plastically according to their positions along the regional climate gradient, as well as locally between matched locations. All species exhibited highly plastic responses and stress-tolerant behaviours, especially in terms of seedling survival during summer drought. However, no evidence of local adaptation was detected in any of the locations, where local individuals never performed better than those from other sites. In some sites, both germination and seedling recruitment were higher irrespective of parent plant origin.
Conclusions The lack of local adaptation to drought displayed at the regeneration stage indicates limited capacity for in situ genetic response to new climate scenarios. Nevertheless, a plastic response along the climatic gradient does suggest a wider species-level capacity to enable these edaphic specialists to cope with increasing aridity over coming decades.
INTRODUCTION
Local adaptation and phenotypic plasticity are important mechanisms that allow plant species to cope in situ with climate change rather than face local extinction and/or geographical range shifts imposed by rapid environmental change (Parmesan, 2006; Lavergne et al., 2010; Parmesan and Hanley, 2015). In situ adjustments to changing environments are particularly critical for plant species with limited dispersal and/or pollen flow (Thuiller et al., 2005), and for plants that inhabit island-like habitats, such as mountains (Byars et al., 2007; Gonzalo-Turpin and Hazard, 2009; Bastida et al., 2014) or restrictive soil outcrops [e.g. serpentine soils (Damschen et al., 2012) and gypsum soils (Matesanz et al., 2009)], where shifts in distribution are unlikely.
2 Local adaptation is a condition in which genotypes exhibit higher fitness locally compared with genotypes from other habitats or locations (Kawecki and Ebert, 2004). This type of genetic differentiation is well documented in plants (Galloway and Fenster, 2000; Volis et al., 2002; Etterson, 2004; Hereford, 2009; Roy et al., 2015) and it is considered a key mechanism that underlies evolutionary potential, with important implications for conservation (Linhart and Grant, 1996). Changes in the environment inhabited by a species can lead to local adaptation if they impose new local selective constraints on traits related to fitness, thereby leading to genetically based evolutionary shifts that extend beyond phenotypic plasticity (Davis et al., 2005; Gienapp et al., 2008). In addition, plant populations that inhabit areas with temporally variable and unpredictable environmental conditions can experience different selective pressures throughout time and even during their life cycle that promote contrasting responses, which are usually mediated by phenotypic plasticity (Sultan, 2000; Alpert and Simms, 2002; Pigliucci, 2005). Local adaptation is more likely when gene flow is limited, but particularly when tolerance and phenotypic plasticity cannot generate optimal phenotypes for each specific environmental condition (Kawecki and Ebert, 2004). Paradoxically, fragmented and isolated populations should be particularly prone to local adaptation due to limited gene flow, whereas small populations are less likely to exhibit local adaption due to increased genetic drift (Leimu and Fischer, 2008).
3 Gypsum soils comprise a model example of an island-like habitat. This particular soil type has a highly restrictive filtering effect on plant development due to its physical and chemical properties (Escudero et al., 2015). These soils have given rise to a singular assembly of plants, most of which are narrow endemics, including a high percentage of threatened or endangered species at different spatial scales according to the International Union for Conservation of Nature (IUCN) criteria (for details of the current situation in Spain, see Martínez-Hernández et al., 2011).
4 These habitats are experiencing the effects of several global change drivers, including man-driven fragmentation due to the intensification of agriculture (Pueyo et al., 2008; Matesanz et al., 2009), but the main concern is increased aridity due to warming (Christensen et al., 2007). Therefore, it is necessary to determine the extent to which gypsophytes can cope with climate warming. Colonization of new gypsum habitats is highly unlikely due to the general absence of efficient dispersal among these plants (Escudero et al., 1997) and because of the island-like structure of this habitat type. Thus, in situ processes related to phenotypic plasticity and local adaptation may help to ensure their persistence in future conditions.
Multisite reciprocal sowing experiments along geographical or environmental gradients are probably the most efficient approach for evaluating local adaptation under field conditions (Nuismer and Gandon, 2008). These experiments substitute space for time to infer past adaptations to climate, thereby allowing the consideration of evolution and the adaptive potential of species when investigating responses to climate change (Lavergne et al., 2010).
In addition, reciprocal sowing experiments allow us to observe the ability of plants to exhibit differences in their average performance in contrasting climate conditions (in other locations), which we refer to as phenotypic plasticity in the following (sensuLázaro-Nogal et al., 2015). In the present study we used this approach to test the hypothesis that dominant gypsum-specialist perennials exhibit local adaptation and phenotypic plasticity. All of these species are restricted to gypsum soils but they differ in terms of their distribution range from wide to narrow distributions (Escudero et al., 2015). Widely distributed gypsophytes are considered specialist taxa based on the chemical contents of their leaves (higher concentrations of S, Ca, Mg, N, P and total ash), which are specifically adapted to gypsum, whereas narrow types are mostly stress-tolerant species without specific adaptations to gypsum soils (Palacio et al., 2007, 2014). The seeds were sown reciprocally among locations, which covered a wide climatic gradient. First, seeds were collected from these sowing locations and other selected matched locations, which were located in the same position on the climatic gradient. This made our design more robust against potential effects related to highly idiosyncratic local climatic events, which can affect plants and their offspring in a location irrespective of the average climatic position. In addition, seeds were collected during two consecutive years and they were sown in the following growing season because plant performance can vary dramatically between years in these stressful environments (Olano et al., 2011). We focused on earlier life stages (from seeds to 4-year seedlings), which have rarely been considered in studies that have examined climate change influence on plant biology (Parmesan and Hanley, 2015). However, they are a critical phase in the life history of plant populations (Kitajima and Fenner, 2000; Donohue et al., 2010), particularly for seasonally drought-prone, Mediterranean-climate environments (Escudero et al., 1999, 2000; Padilla and Pugnaire, 2007).
In these gypsum habitats, higher germination rates are expected as the autumn and winter temperatures increase, with higher growth rates and survival in rainier locations where drought stress is lower (Palacio et al., 2007; Escudero et al., 2015), which (irrespective of the seed origins) would allow us to conclude that the study species are not locally adapted. By contrast, better performance at a given site by a local relative of foreign plants could be interpreted as evidence of local adaptation (Kawecki and Ebert, 2004). In addition, a significant effect of the sowing location on the performance of a species would indicate a plastic response to climatic variability. For two narrow endemics that are restricted to higher and rainier parts of the gradient, a reciprocal sowing experiment including locations outside their geographical range may determine whether they could potentially handle variations in temperature and drought stress. In fact, determining the performance of each study species under contrasting stress levels allowed us to predict its ability to cope with future warmer and drier conditions. Moreover, the combination of all species responses allowed us to scale up to the community level consequences in a future warming scenario.
MATERIALS AND METHODS
Study area
This study was conducted within the extensive gypsum steppes of central Spain. This landscape encompasses a vast territory in the Tagus river valley, which ranges from the southernmost tip of Madrid province to the piedmont of the Serranía de Cuenca at the most eastern point (Fig. 1).
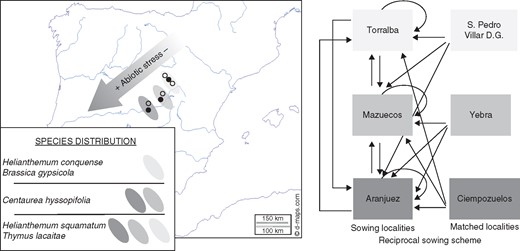
Map of the Iberian Peninsula showing the locations of the sites where seeds were collected (open and black circles) and sown (black circles). The distribution ranges of the study species along gypsum outcrops in the Tagus river valley and the scheme employed in the reciprocal sowing experiment are also shown.
Gypsum outcrops and massive gypsisols are dominant in this region, where the percentage of gypsum in the soil is usually well above 70 %. The climate and altitude vary from the northeast, which is wet and cold, to the southwest, which is dry and warm, with a steep environmental gradient (Table 1). The vegetation comprises a diverse array of gypsophile scrubs interspersed with a rich annual community (Ferrandis et al., 2005).
Geographical position and climate conditions (Ninyerola et al., 2005) of the selected locations among gypsum outcrops in the Tagus valley. Seeds were collected from all locations and were sown at locations shown in capitals. See Fig. 1 for further details of the study area, study species distribution and reciprocal sowing scheme
| . | . | Height (m) . | Minimum/mean/maximum annual temperature (°C) . | Mean annual rainfall (mm) . |
|---|---|---|---|---|
| ARANJUEZ | 40°02′ N, 3°32′ W | 590 | 8/15/23 | 419 |
| Ciempozuelos | 40°08′ N, 3°36′ W | 568 | 8/15/22 | 418 |
| MAZUECOS | 40°16′ N, 3°00′ W | 734 | 7/14/21 | 477 |
| Yebra | 40°20′ N, 2°56′ W | 729 | 7/14/21 | 489 |
| TORRALBA | 40°18′ N, 2°17′ W | 920 | 6/13/20 | 611 |
| San Pedro P. | 40°25′ N, 2°23′ W | 861 | 6/13/19 | 604 |
| Villar D. G. | 40°14′ N, 2°18′ W | 932 | 6/13/20 | 600 |
| . | . | Height (m) . | Minimum/mean/maximum annual temperature (°C) . | Mean annual rainfall (mm) . |
|---|---|---|---|---|
| ARANJUEZ | 40°02′ N, 3°32′ W | 590 | 8/15/23 | 419 |
| Ciempozuelos | 40°08′ N, 3°36′ W | 568 | 8/15/22 | 418 |
| MAZUECOS | 40°16′ N, 3°00′ W | 734 | 7/14/21 | 477 |
| Yebra | 40°20′ N, 2°56′ W | 729 | 7/14/21 | 489 |
| TORRALBA | 40°18′ N, 2°17′ W | 920 | 6/13/20 | 611 |
| San Pedro P. | 40°25′ N, 2°23′ W | 861 | 6/13/19 | 604 |
| Villar D. G. | 40°14′ N, 2°18′ W | 932 | 6/13/20 | 600 |
Geographical position and climate conditions (Ninyerola et al., 2005) of the selected locations among gypsum outcrops in the Tagus valley. Seeds were collected from all locations and were sown at locations shown in capitals. See Fig. 1 for further details of the study area, study species distribution and reciprocal sowing scheme
| . | . | Height (m) . | Minimum/mean/maximum annual temperature (°C) . | Mean annual rainfall (mm) . |
|---|---|---|---|---|
| ARANJUEZ | 40°02′ N, 3°32′ W | 590 | 8/15/23 | 419 |
| Ciempozuelos | 40°08′ N, 3°36′ W | 568 | 8/15/22 | 418 |
| MAZUECOS | 40°16′ N, 3°00′ W | 734 | 7/14/21 | 477 |
| Yebra | 40°20′ N, 2°56′ W | 729 | 7/14/21 | 489 |
| TORRALBA | 40°18′ N, 2°17′ W | 920 | 6/13/20 | 611 |
| San Pedro P. | 40°25′ N, 2°23′ W | 861 | 6/13/19 | 604 |
| Villar D. G. | 40°14′ N, 2°18′ W | 932 | 6/13/20 | 600 |
| . | . | Height (m) . | Minimum/mean/maximum annual temperature (°C) . | Mean annual rainfall (mm) . |
|---|---|---|---|---|
| ARANJUEZ | 40°02′ N, 3°32′ W | 590 | 8/15/23 | 419 |
| Ciempozuelos | 40°08′ N, 3°36′ W | 568 | 8/15/22 | 418 |
| MAZUECOS | 40°16′ N, 3°00′ W | 734 | 7/14/21 | 477 |
| Yebra | 40°20′ N, 2°56′ W | 729 | 7/14/21 | 489 |
| TORRALBA | 40°18′ N, 2°17′ W | 920 | 6/13/20 | 611 |
| San Pedro P. | 40°25′ N, 2°23′ W | 861 | 6/13/19 | 604 |
| Villar D. G. | 40°14′ N, 2°18′ W | 932 | 6/13/20 | 600 |
Study species
We studied five dominant and endemic gypsophyte perennial plants in Central Spain. Thymus lacaitae (Lamiaceae) is present from 200 to 900 m above sea level on gypsum outcrops in Central Spain. Helianthemum squamatum (Cistaceae) occurs in many of the gypsum outcrops (40–900 m) in the Iberian Peninsula and North Africa. Centaurea hyssopifolia (Asteraceae) is an endemic species found at altitudes ranging between 400 and 900 m in Central Spain, as well as in small patches on outcrops near the Mediterranean coast (southeast Spain) and in the Ebro valley (northeast Spain). Finally, Helianthemum marifolium ssp. conquense (Cistaceae) and Brassica repanda ssp. gypsicola (Brassicaceae) are Iberian endemics found in the gypsum steppes of Guadalajara and Cuenca provinces. On our stress climate gradient, T. lacaitae and H. squamatum occurred throughout the study area, C. hyssopifolia were distributed in the intermediate and low zones, and H. conquense and B. gypsicola were present only in the mildest and easternmost part of the gradient [Fig. 1; see Mota et al. (2011) for detailed information about the distributions of these species]. The first three species can be considered regionally dominant gypsophytes, whereas the latter two are clear examples of narrowly distributed gypsophytes (Escudero et al., 2015).
Reciprocal sowing design
We selected three small areas across a geographical abiotic stress gradient defined by temperature and precipitation. We selected a sowing location within each area and matched these sowing sites with one or two locations where we collected seeds but did not conduct sowing experiments (Table 1, Fig. 1). The longest linear distance between our sowing sites was 115·9 km from Aranjuez and Torralba, while Mazuecos was 56·4 km northeast of Aranjuez and 61·9 km southwest of Torralba. The matched location for Aranjuez was Ciempozuelos and Yebra was the match for Mazuecos. Torralba had two matched locations, i.e. San Pedro Palmiches and Villar de Domingo García, because not all of the species were present within a single site. We collected mature fruits from at least 50 randomly selected individuals belonging to each species at each site and the matched locations between June and August in 2007 and 2008. Collecting the seeds from a high number of healthy mother plants in two different years allowed us to dilute maternal effects as a confounding factor. Seeds were stored in paper bags at room temperature until the sowing experiments. Helianthemum squamatum and H. conquense were scarified mechanically with fine-grained sandpaper in order to disrupt the primary dormancy imposed by their hard coats (Escudero et al. 1997).
We performed a reciprocal sowing experiment where seeds were sown in their original locations as well as at other sites where the plants did or did not occur naturally (see the sowing design in Fig. 1). In each of the three sowing sites, five plots were established on bare areas. Each plot comprised up to 20 quadrats (25×25 cm) placed along two parallel transects, to which all possible combinations of species and seed origins were assigned randomly (Fig. 1). Thus, each combination of sowing and original location was replicated in five plots, except for T. lacaitae, which only had three replicates at each sowing location due to the relatively low number of available seeds. This design was not fully complete in the case of C. hyssopifolia because there were insufficient seeds from Aranjuez and Ciempozuelos for sowing in Torralba, as well as seeds from Mazuecos for sowing in Aranjuez. During October 2007, the seeds were sown using a transparent plastic sheet measuring 25×25 cm, with 1-cm holes (n = 25) spaced 5 cm apart. We conducted a second sowing during February 2009 at the same plots and prepared a complementary set of holes (n = 16) using the free spaces between previously used holes (Supplementary Data Appendix S1). The number of seeds sown per hole varied according to seed size and seed availability per species. Two seeds were sown per hole in 2007, except that five seeds per hole were used for T. lacaitae. Five seeds per hole were sown in 2009, except that we used three seeds per hole for C. hyssopifolia. Seeds were sown ∼5 mm beneath the soil surface and covered with sieved local topsoil. Seeds were sown under field conditions without removing or clipping the natural vegetation.
Germination rates were recorded each month until summer 2008 and every 2–3 months during the remainder of the study period using the same grid as that employed for sowing. Thus, we could differentiate our seedlings from those that potentially emerged from the seed bank. The second and subsequent seedlings that emerged at each point were recorded to quantify germination and then removed to avoid inter-seedling competition, so the number of germinations and the number of periodically observed seedlings were not the same (Table 2). In each survey, we also recorded the number of leaves of the seedlings, and survival. Each plot was visited until May 2011, when the seeds in the first and second experiments had completed their fourth and third germination (autumn–spring) periods, respectively.
Seeds and seedling performance of each species. Data represent summarized information for the whole study period and from the three locations where reciprocal sowing was performed. A variable number (two to five), depending on the species, was sown at each sowing point, but only one seedling per sowing point was left to observe growth and survival (studied seedlings). Germination timing indicates the percentage of recorded germination that occured during the first, second and third year since sowing
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown* | 9840 | 8060 | 4410 | 3900 | 3900 |
| Number germinated | 564 | 632 | 456 | 596 | 572 |
| Germination rate (%) | 5·7 | 7·8 | 10·34 | 15·3 | 14·7 |
| Germination timing (%, 1st/2nd/3rd years) | 96/4/0 | 47/34/19 | 21/64/15 | 97/2/1 | 54/36/10 |
| Studied seedlings | 300 | 460 | 398 | 318 | 430 |
| Living seedlings after first summer | 118 | 174 | 167 | 112 | 192 |
| First summer survival (%) | 39·3 | 37·8 | 42·0 | 35·2 | 44·7 |
| Final living seedlings | 2 | 17 | 27 | 7 | 14 |
| Final survival (%) | 0·7 | 3·7 | 6·8 | 2·2 | 3·2 |
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown* | 9840 | 8060 | 4410 | 3900 | 3900 |
| Number germinated | 564 | 632 | 456 | 596 | 572 |
| Germination rate (%) | 5·7 | 7·8 | 10·34 | 15·3 | 14·7 |
| Germination timing (%, 1st/2nd/3rd years) | 96/4/0 | 47/34/19 | 21/64/15 | 97/2/1 | 54/36/10 |
| Studied seedlings | 300 | 460 | 398 | 318 | 430 |
| Living seedlings after first summer | 118 | 174 | 167 | 112 | 192 |
| First summer survival (%) | 39·3 | 37·8 | 42·0 | 35·2 | 44·7 |
| Final living seedlings | 2 | 17 | 27 | 7 | 14 |
| Final survival (%) | 0·7 | 3·7 | 6·8 | 2·2 | 3·2 |
These values are slightly below the initial numbers of sown seeds due to damage in some plots, which was caused mainly by rabbits.
Seeds and seedling performance of each species. Data represent summarized information for the whole study period and from the three locations where reciprocal sowing was performed. A variable number (two to five), depending on the species, was sown at each sowing point, but only one seedling per sowing point was left to observe growth and survival (studied seedlings). Germination timing indicates the percentage of recorded germination that occured during the first, second and third year since sowing
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown* | 9840 | 8060 | 4410 | 3900 | 3900 |
| Number germinated | 564 | 632 | 456 | 596 | 572 |
| Germination rate (%) | 5·7 | 7·8 | 10·34 | 15·3 | 14·7 |
| Germination timing (%, 1st/2nd/3rd years) | 96/4/0 | 47/34/19 | 21/64/15 | 97/2/1 | 54/36/10 |
| Studied seedlings | 300 | 460 | 398 | 318 | 430 |
| Living seedlings after first summer | 118 | 174 | 167 | 112 | 192 |
| First summer survival (%) | 39·3 | 37·8 | 42·0 | 35·2 | 44·7 |
| Final living seedlings | 2 | 17 | 27 | 7 | 14 |
| Final survival (%) | 0·7 | 3·7 | 6·8 | 2·2 | 3·2 |
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown* | 9840 | 8060 | 4410 | 3900 | 3900 |
| Number germinated | 564 | 632 | 456 | 596 | 572 |
| Germination rate (%) | 5·7 | 7·8 | 10·34 | 15·3 | 14·7 |
| Germination timing (%, 1st/2nd/3rd years) | 96/4/0 | 47/34/19 | 21/64/15 | 97/2/1 | 54/36/10 |
| Studied seedlings | 300 | 460 | 398 | 318 | 430 |
| Living seedlings after first summer | 118 | 174 | 167 | 112 | 192 |
| First summer survival (%) | 39·3 | 37·8 | 42·0 | 35·2 | 44·7 |
| Final living seedlings | 2 | 17 | 27 | 7 | 14 |
| Final survival (%) | 0·7 | 3·7 | 6·8 | 2·2 | 3·2 |
These values are slightly below the initial numbers of sown seeds due to damage in some plots, which was caused mainly by rabbits.
The edaphic properties of soils (pH, conductivity, total nitrogen and total phosphorus contents) were determined using standard soil analysis procedures (Escudero et al., 2004) with soil samples (top 7·5 cm of soil) collected from bare ground located near each plot at each of the three sowing locations in July 2007.
Data analysis
We tested the variability of soil properties relative to the sowing location using one-way ANOVA with a post hoc Tukey test to detect differences between sites. We used generalized linear mixed models (GLMMs) to test the effects of the original location (up to six locations per species) and sowing location (three locations) on the germination of seeds (germinated or not germinated) and growth rate (number of leaves/days since germination), survival after the first summer (live or dead) and lifespan of studied seedlings. The sowing experiment was performed twice (seeds were collected and sown in two consecutive years), so we compared the results obtained from the two experiments by including year as a two-level (2007 and 2008) fixed factor. Each two-way interaction among fixed factors was included in the models. (three to five plots for each species at each sowing location) was considered to be a random factor, which was nested in the sowing location.
Germination and survival models were fitted by assuming a binomial error distribution and logit link function. For lifespan, a Poisson distribution and logit link function were selected. Finally, for growth rate, a gamma distribution and inverse/logit link function were specified for model fitting. Data related to the growth rate of T. lacaitae were logarithmically transformed in order to improve the model fit. The residuals were graphically inspected for all of the models. The contributions of fixed factors to the models were tested by analysis of deviance using Wald χ2 tests. In addition, marginal R2 (R2m) and/or conditional R2 (R2c) were calculated as indicators of the variability explained by all of the fixed factors and by the whole model, respectively. All of the analyses were conducted using the lme4 package (Bates et al., 2011) in R 3. 2. 2 (R Development Core Team, 2015).
RESULTS
The amount and timing of germination varied markedly between species (Table 2). Germination rate varied from 15·3 % for H. conquense to 5·7 % for H. squamatum, while survival after the first summer ranged from 35·2 % for the seedlings of H. conquense to 44·7 % in B. gypsicola (Table 2). This percentage decreased greatly towards the end of the study, when it ranged from 0·7 % in H. squamatum to 6·8 % in C. hyssopifolia (Table 2).
There were only very small differences in the soil nitrogen and phosphorus contents, with higher values in the easternmost sowing site, but there were no significant differences among the other soil variables (Supplementary Data Appendix S2).
Germination
We observed no improvements in the germination performance of any species when they were sown at their original locations, and thus we found no evidence of local adaptation during this life stage (Fig. 2). However, there was a complex pattern of variation in germination behaviour among years, sowing sites and seeds with different origins (Table 3). In addition, these patterns were species-dependent, i.e. the germination of H. squamatum seeds was closely related to the climatic gradient, whereby seeds from cold and moist sites germinated at a lower rate than seeds from warmer sites, and regardless of their origin the seed germination rate increased from cold to warmer sites (Fig. 2). Thymus lacaitae seeds with a higher germination rate came from the coldest edge of the gradient, but their germination rate was higher in the intermediate and warm sites (Fig. 2). The germination rate of C. hyssopifolia seeds varied locally, but seeds from all sites had higher germination rates at the warm sowing locations (Fig. 2). Helianthemum conquense seeds from the two original matched locations exhibited opposite germination patterns, with better germination at the intermediate site on the gradient, but a higher germination rate at the cooler edge (Table 3). Finally, the germination rate of B. gypsicola differed significantly between the original matched sites, and there was also an increase in the germination rate from cool to warm sowing sites (Fig. 2).
GLMM results showing the effects of year, sowing and origin location and their interactions on germination of the five gypsophytes. Plot was included as a random factor nested in sowing location. Wald χ2 values are indicated with number of degrees of freedom in parentheses. All models were fitted by assuming a binomial error distribution and a logit link function. R2m and R2c indicate the variability explained by all fixed factors and the whole model, respectively
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 9840 | 8060 | 4410 | 3900 | 3900 |
| R2m/R2c | 0·34/0·53 | 0·25/0·26 | 0·28/0·33 | 0·04/0·16 | 0·16/0·19 |
| Year (Y) | 72·0 (1)*** | 7·1 (1)** | 149·2 (1)*** | 24·5 (1)*** | 81·1 (1)*** |
| Sowing location (S) | 7·9 (2)* | 11·0 (2)** | 24·02 (2)*** | 1·4 (2) | 17·2 (2)*** |
| Origin (O) | 49·8 (5)*** | 122·8 (5)*** | 14·1 (3)** | 0·5 (1) | 9·03 (1)** |
| O × S | 22·1 (10)* | 52·7 (10)*** | – | 9·2 (2)* | 4·1 (2) |
| O × Y | 10·2 (5) | 28·7 (5)*** | 8·5 (3)* | 0·3 (1) | 0·1 (1) |
| S × Y | 13·6 (2)** | 56·1 (2)*** | 17·9 (2)*** | 2·2 (2) | 42·6 (2)*** |
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 9840 | 8060 | 4410 | 3900 | 3900 |
| R2m/R2c | 0·34/0·53 | 0·25/0·26 | 0·28/0·33 | 0·04/0·16 | 0·16/0·19 |
| Year (Y) | 72·0 (1)*** | 7·1 (1)** | 149·2 (1)*** | 24·5 (1)*** | 81·1 (1)*** |
| Sowing location (S) | 7·9 (2)* | 11·0 (2)** | 24·02 (2)*** | 1·4 (2) | 17·2 (2)*** |
| Origin (O) | 49·8 (5)*** | 122·8 (5)*** | 14·1 (3)** | 0·5 (1) | 9·03 (1)** |
| O × S | 22·1 (10)* | 52·7 (10)*** | – | 9·2 (2)* | 4·1 (2) |
| O × Y | 10·2 (5) | 28·7 (5)*** | 8·5 (3)* | 0·3 (1) | 0·1 (1) |
| S × Y | 13·6 (2)** | 56·1 (2)*** | 17·9 (2)*** | 2·2 (2) | 42·6 (2)*** |
P ≤ 0·05;
P ≤ 0·01;
P ≤ 0·001.
GLMM results showing the effects of year, sowing and origin location and their interactions on germination of the five gypsophytes. Plot was included as a random factor nested in sowing location. Wald χ2 values are indicated with number of degrees of freedom in parentheses. All models were fitted by assuming a binomial error distribution and a logit link function. R2m and R2c indicate the variability explained by all fixed factors and the whole model, respectively
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 9840 | 8060 | 4410 | 3900 | 3900 |
| R2m/R2c | 0·34/0·53 | 0·25/0·26 | 0·28/0·33 | 0·04/0·16 | 0·16/0·19 |
| Year (Y) | 72·0 (1)*** | 7·1 (1)** | 149·2 (1)*** | 24·5 (1)*** | 81·1 (1)*** |
| Sowing location (S) | 7·9 (2)* | 11·0 (2)** | 24·02 (2)*** | 1·4 (2) | 17·2 (2)*** |
| Origin (O) | 49·8 (5)*** | 122·8 (5)*** | 14·1 (3)** | 0·5 (1) | 9·03 (1)** |
| O × S | 22·1 (10)* | 52·7 (10)*** | – | 9·2 (2)* | 4·1 (2) |
| O × Y | 10·2 (5) | 28·7 (5)*** | 8·5 (3)* | 0·3 (1) | 0·1 (1) |
| S × Y | 13·6 (2)** | 56·1 (2)*** | 17·9 (2)*** | 2·2 (2) | 42·6 (2)*** |
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 9840 | 8060 | 4410 | 3900 | 3900 |
| R2m/R2c | 0·34/0·53 | 0·25/0·26 | 0·28/0·33 | 0·04/0·16 | 0·16/0·19 |
| Year (Y) | 72·0 (1)*** | 7·1 (1)** | 149·2 (1)*** | 24·5 (1)*** | 81·1 (1)*** |
| Sowing location (S) | 7·9 (2)* | 11·0 (2)** | 24·02 (2)*** | 1·4 (2) | 17·2 (2)*** |
| Origin (O) | 49·8 (5)*** | 122·8 (5)*** | 14·1 (3)** | 0·5 (1) | 9·03 (1)** |
| O × S | 22·1 (10)* | 52·7 (10)*** | – | 9·2 (2)* | 4·1 (2) |
| O × Y | 10·2 (5) | 28·7 (5)*** | 8·5 (3)* | 0·3 (1) | 0·1 (1) |
| S × Y | 13·6 (2)** | 56·1 (2)*** | 17·9 (2)*** | 2·2 (2) | 42·6 (2)*** |
P ≤ 0·05;
P ≤ 0·01;
P ≤ 0·001.
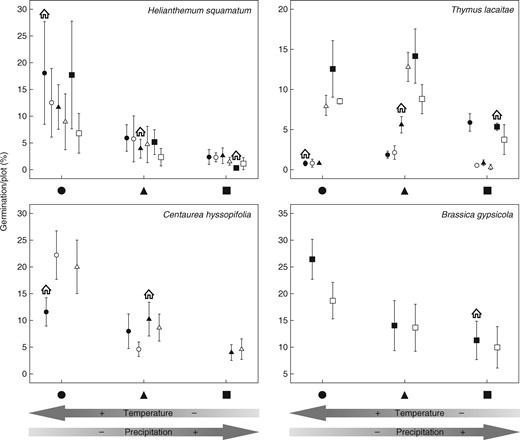
Percentage germination per plot (mean ± s.e.) at the three sowing locations grouped by seed origin (black circles, triangles and squares represent the three sowing locations and open circles, triangles and squares represent matched locations). Seeds sown at their home location are indicated by the house symbol. Better performance by seeds at their home site is indicative of local adaptation, while differences in the performance of seeds from a location along the climatic gradient are indicative of population phenotypic plasticity. Arrows at the bottom of the figure indicate the position of each location on the climatic gradient.
Seedling growth
We detected no evidence of local adaptation in this life stage because seedlings did not exhibit greater growth rates at their original sites compared with the same species from other areas. However, some growth rate variability was observed between years, except in T. lacaitae. The growth rate varied according to the origin, except for C. hyssopifolia. Furthermore, the growth rate varied according to the sowing site, but only in the case of H. squamatum, which had a higher growth rate at the intermediate sowing site (Table 4, Fig. 3).
GLMM results showing the effects of year, sowing and origin location and their interactions on growth rates of the five gypsophytes. Plot was included as a random factor nested in sowing location. Wald χ2 values are indicated with number of degrees of freedom in parentheses. All models were fitted by assuming a gamma error distribution and an inverse/logit link function. Number of observations (n, studied seedlings) is also shown for each model
| . | Helianthemum squamatum . | Thymus lacaitae Log10(x+1) . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| Year (Y) | 116·3 (1)*** | 0·0 (1) | 6·3 (1)* | 237·7 (1)*** | 40·8 (1)*** |
| Sowing location (S) | 7·2 (2)* | 3·9 (2) | 0·1 (2) | 0·2 (2) | 1·4 (2) |
| Origin (O) | 12·9 (5)* | 5·6 (5)* | 1·6 (3) | 5·1 (1)* | 4·0 (1)* |
| O × S | 13·0 (10) | 20·6 (10) | — | 34·0 (2)*** | 3·8 (2) |
| O × Y | 4·0 (5) | 8·0 (5) | 6·3 (3) | 0·03 (1) | 0·2 (1) |
| S × Y | 0·5 (2) | 3·4 (2) | 0·9 (2) | 7·6 (2)* | 0·2 (2) |
| . | Helianthemum squamatum . | Thymus lacaitae Log10(x+1) . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| Year (Y) | 116·3 (1)*** | 0·0 (1) | 6·3 (1)* | 237·7 (1)*** | 40·8 (1)*** |
| Sowing location (S) | 7·2 (2)* | 3·9 (2) | 0·1 (2) | 0·2 (2) | 1·4 (2) |
| Origin (O) | 12·9 (5)* | 5·6 (5)* | 1·6 (3) | 5·1 (1)* | 4·0 (1)* |
| O × S | 13·0 (10) | 20·6 (10) | — | 34·0 (2)*** | 3·8 (2) |
| O × Y | 4·0 (5) | 8·0 (5) | 6·3 (3) | 0·03 (1) | 0·2 (1) |
| S × Y | 0·5 (2) | 3·4 (2) | 0·9 (2) | 7·6 (2)* | 0·2 (2) |
P ≤ 0·05;
P ≤ 0·001.
GLMM results showing the effects of year, sowing and origin location and their interactions on growth rates of the five gypsophytes. Plot was included as a random factor nested in sowing location. Wald χ2 values are indicated with number of degrees of freedom in parentheses. All models were fitted by assuming a gamma error distribution and an inverse/logit link function. Number of observations (n, studied seedlings) is also shown for each model
| . | Helianthemum squamatum . | Thymus lacaitae Log10(x+1) . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| Year (Y) | 116·3 (1)*** | 0·0 (1) | 6·3 (1)* | 237·7 (1)*** | 40·8 (1)*** |
| Sowing location (S) | 7·2 (2)* | 3·9 (2) | 0·1 (2) | 0·2 (2) | 1·4 (2) |
| Origin (O) | 12·9 (5)* | 5·6 (5)* | 1·6 (3) | 5·1 (1)* | 4·0 (1)* |
| O × S | 13·0 (10) | 20·6 (10) | — | 34·0 (2)*** | 3·8 (2) |
| O × Y | 4·0 (5) | 8·0 (5) | 6·3 (3) | 0·03 (1) | 0·2 (1) |
| S × Y | 0·5 (2) | 3·4 (2) | 0·9 (2) | 7·6 (2)* | 0·2 (2) |
| . | Helianthemum squamatum . | Thymus lacaitae Log10(x+1) . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| Year (Y) | 116·3 (1)*** | 0·0 (1) | 6·3 (1)* | 237·7 (1)*** | 40·8 (1)*** |
| Sowing location (S) | 7·2 (2)* | 3·9 (2) | 0·1 (2) | 0·2 (2) | 1·4 (2) |
| Origin (O) | 12·9 (5)* | 5·6 (5)* | 1·6 (3) | 5·1 (1)* | 4·0 (1)* |
| O × S | 13·0 (10) | 20·6 (10) | — | 34·0 (2)*** | 3·8 (2) |
| O × Y | 4·0 (5) | 8·0 (5) | 6·3 (3) | 0·03 (1) | 0·2 (1) |
| S × Y | 0·5 (2) | 3·4 (2) | 0·9 (2) | 7·6 (2)* | 0·2 (2) |
P ≤ 0·05;
P ≤ 0·001.
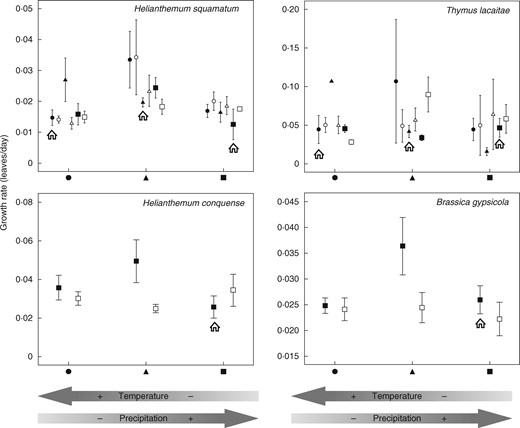
Growth rate for each plot (mean ± s.e.) at the three sowing locations grouped by seed origin (black circles, triangles and squares represent the three sowing locations and open circles, triangles and squares represent matched locations). Seeds sown at their home location are indicated by the house symbol. Better performance by seeds at their home site is indicative of local adaptation, while differences in the performance of seeds from a location along the climatic gradient are indicative of population phenotypic plasticity. The arrows at the bottom of the figure indicate the position of each location on the climatic gradient.
Seedling lifespan and survival
Seedling survival after the first summer was almost 40 % (Table 2), which was related to the sowing site (Table 5). Helianthemum squamatum, T. lacaitae and C. hyssopifolia produced a higher number of living seedlings at the warm, westernmost side of the gradient. In addition, seedling survival was related to seed origin in these more arid conditions, but not significantly. Seedling survival in H. squamatum tended to increase from cool to warm seed origins, whereas T. lacaitae and C. hyssopifolia exhibited the opposite pattern (Fig. 4). This trend was not evident in the remaining sowing sites and thus there was no net effect of seed origin on survival in these species. Evidence of local adaptation was not detected because the survival of seedlings in their local area was not greater than that of individuals sown elsewhere (Fig. 4). For H. conquense and B. gypsicola, the site with the highest number of living seedlings after the first summer varied between the study years (Table 6), but we observed no advantage in seedlings grown at their home sites compared with seedlings growing outside the area where they occurred naturally.
GLMM results showing the effects of year, sowing and origin location and their interactions on seedling survival after the first summer drought for the five gypsophytes. Plot was included as a random factor nested in sowing location. Wald χ2 values are indicated with number of degrees of freedom in parentheses. All models were fitted by assuming a binomial error distribution and a logit link function. R2m and R2c indicate the variability explained by all fixed factors and the whole model, respectively. Number of observations (n, studied seedlings) is also shown for each model
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| R2m/R2c | 0·89/0·92 | 0·86/0·87 | 0·17/0·32 | 0·12/0·30 | 0·31/0·36 |
| Year (Y) | 0·1 (1) | 13·0 (1)*** | 0·3 (1) | 0·1 (1) | 27·6 (1)*** |
| Sowing location (S) | 6·0 (2)* | 11·3 (3)* | 8·2 (2)* | 0·5 (2) | 6·7 (1) |
| Origin (O) | 8·5 (5) | 4·5 (6) | 1·6 (3) | 0·0 (1) | 1·9 (1) |
| O × S | 15·4 (10) | 8·7 (10) | – | 2·1 (2) | 0·5 (2) |
| O × Y | 3·5 (5) | 7·4 (5) | 1·8 (3) | 5·0 (1)* | 1·3 (1) |
| S × Y | 8·9 (2)* | 39·0 (2)*** | 1·2 (2) | 14·2 (2)*** | 38·5 (2)*** |
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| R2m/R2c | 0·89/0·92 | 0·86/0·87 | 0·17/0·32 | 0·12/0·30 | 0·31/0·36 |
| Year (Y) | 0·1 (1) | 13·0 (1)*** | 0·3 (1) | 0·1 (1) | 27·6 (1)*** |
| Sowing location (S) | 6·0 (2)* | 11·3 (3)* | 8·2 (2)* | 0·5 (2) | 6·7 (1) |
| Origin (O) | 8·5 (5) | 4·5 (6) | 1·6 (3) | 0·0 (1) | 1·9 (1) |
| O × S | 15·4 (10) | 8·7 (10) | – | 2·1 (2) | 0·5 (2) |
| O × Y | 3·5 (5) | 7·4 (5) | 1·8 (3) | 5·0 (1)* | 1·3 (1) |
| S × Y | 8·9 (2)* | 39·0 (2)*** | 1·2 (2) | 14·2 (2)*** | 38·5 (2)*** |
P ≤ 0·05;
P ≤ 0·001.
GLMM results showing the effects of year, sowing and origin location and their interactions on seedling survival after the first summer drought for the five gypsophytes. Plot was included as a random factor nested in sowing location. Wald χ2 values are indicated with number of degrees of freedom in parentheses. All models were fitted by assuming a binomial error distribution and a logit link function. R2m and R2c indicate the variability explained by all fixed factors and the whole model, respectively. Number of observations (n, studied seedlings) is also shown for each model
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| R2m/R2c | 0·89/0·92 | 0·86/0·87 | 0·17/0·32 | 0·12/0·30 | 0·31/0·36 |
| Year (Y) | 0·1 (1) | 13·0 (1)*** | 0·3 (1) | 0·1 (1) | 27·6 (1)*** |
| Sowing location (S) | 6·0 (2)* | 11·3 (3)* | 8·2 (2)* | 0·5 (2) | 6·7 (1) |
| Origin (O) | 8·5 (5) | 4·5 (6) | 1·6 (3) | 0·0 (1) | 1·9 (1) |
| O × S | 15·4 (10) | 8·7 (10) | – | 2·1 (2) | 0·5 (2) |
| O × Y | 3·5 (5) | 7·4 (5) | 1·8 (3) | 5·0 (1)* | 1·3 (1) |
| S × Y | 8·9 (2)* | 39·0 (2)*** | 1·2 (2) | 14·2 (2)*** | 38·5 (2)*** |
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| R2m/R2c | 0·89/0·92 | 0·86/0·87 | 0·17/0·32 | 0·12/0·30 | 0·31/0·36 |
| Year (Y) | 0·1 (1) | 13·0 (1)*** | 0·3 (1) | 0·1 (1) | 27·6 (1)*** |
| Sowing location (S) | 6·0 (2)* | 11·3 (3)* | 8·2 (2)* | 0·5 (2) | 6·7 (1) |
| Origin (O) | 8·5 (5) | 4·5 (6) | 1·6 (3) | 0·0 (1) | 1·9 (1) |
| O × S | 15·4 (10) | 8·7 (10) | – | 2·1 (2) | 0·5 (2) |
| O × Y | 3·5 (5) | 7·4 (5) | 1·8 (3) | 5·0 (1)* | 1·3 (1) |
| S × Y | 8·9 (2)* | 39·0 (2)*** | 1·2 (2) | 14·2 (2)*** | 38·5 (2)*** |
P ≤ 0·05;
P ≤ 0·001.
GLMM results showing the effects of year, sowing and origin location and their interactions on seedling lifespan of the five gypsophytes. Plot was included as a random factor nested in sowing location. Wald χ2 values are indicated with number of degrees of freedom in parentheses. All models were fitted by assuming a Poisson error distribution and a logit link function. R2m indicates the variability explained by all the fixed factors. Number of observations (n, studied seedlings) is also shown for each model
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| R2m | 0·66 | 0·93 | 0·34 | 0·36 | 0·83 |
| Year (Y) | 1333·4 (1)*** | 5052·8 (1)*** | 935·5 (1)*** | 2138·5 (1)*** | 3317·0 (1)*** |
| Sowing location (S) | 9·9 (2)** | 26·0 (2)*** | 4·6 (2) | 3·2 (2) | 33·5 (2)*** |
| Origin (O) | 323·7 (1)*** | 327·4 (5)*** | 107·3 (3)*** | 1·1 (1) | 223·3 (1)*** |
| O × S | 2004·2 (1)*** | 564·6 (10)*** | — | 325·1 (2)*** | 723·8 (2)*** |
| O × Y | 778·1 (1)*** | 395·7 (5)*** | 740·9 (3)*** | 186·3 (1)*** | 0·4 (1)*** |
| S × Y | 742·7 (1)*** | 4311·8 (2)*** | 1670·4 (2)*** | 3104·5 (2)*** | 1605·3 (1)*** |
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| R2m | 0·66 | 0·93 | 0·34 | 0·36 | 0·83 |
| Year (Y) | 1333·4 (1)*** | 5052·8 (1)*** | 935·5 (1)*** | 2138·5 (1)*** | 3317·0 (1)*** |
| Sowing location (S) | 9·9 (2)** | 26·0 (2)*** | 4·6 (2) | 3·2 (2) | 33·5 (2)*** |
| Origin (O) | 323·7 (1)*** | 327·4 (5)*** | 107·3 (3)*** | 1·1 (1) | 223·3 (1)*** |
| O × S | 2004·2 (1)*** | 564·6 (10)*** | — | 325·1 (2)*** | 723·8 (2)*** |
| O × Y | 778·1 (1)*** | 395·7 (5)*** | 740·9 (3)*** | 186·3 (1)*** | 0·4 (1)*** |
| S × Y | 742·7 (1)*** | 4311·8 (2)*** | 1670·4 (2)*** | 3104·5 (2)*** | 1605·3 (1)*** |
P ≤ 0·01;
P ≤ 0·001.
GLMM results showing the effects of year, sowing and origin location and their interactions on seedling lifespan of the five gypsophytes. Plot was included as a random factor nested in sowing location. Wald χ2 values are indicated with number of degrees of freedom in parentheses. All models were fitted by assuming a Poisson error distribution and a logit link function. R2m indicates the variability explained by all the fixed factors. Number of observations (n, studied seedlings) is also shown for each model
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| R2m | 0·66 | 0·93 | 0·34 | 0·36 | 0·83 |
| Year (Y) | 1333·4 (1)*** | 5052·8 (1)*** | 935·5 (1)*** | 2138·5 (1)*** | 3317·0 (1)*** |
| Sowing location (S) | 9·9 (2)** | 26·0 (2)*** | 4·6 (2) | 3·2 (2) | 33·5 (2)*** |
| Origin (O) | 323·7 (1)*** | 327·4 (5)*** | 107·3 (3)*** | 1·1 (1) | 223·3 (1)*** |
| O × S | 2004·2 (1)*** | 564·6 (10)*** | — | 325·1 (2)*** | 723·8 (2)*** |
| O × Y | 778·1 (1)*** | 395·7 (5)*** | 740·9 (3)*** | 186·3 (1)*** | 0·4 (1)*** |
| S × Y | 742·7 (1)*** | 4311·8 (2)*** | 1670·4 (2)*** | 3104·5 (2)*** | 1605·3 (1)*** |
| . | Helianthemum squamatum . | Thymus lacaitae . | Centaurea hyssopifolia . | Helianthemum conquense . | Brassica gypsicola . |
|---|---|---|---|---|---|
| No. of seeds sown | 300 | 460 | 398 | 318 | 430 |
| R2m | 0·66 | 0·93 | 0·34 | 0·36 | 0·83 |
| Year (Y) | 1333·4 (1)*** | 5052·8 (1)*** | 935·5 (1)*** | 2138·5 (1)*** | 3317·0 (1)*** |
| Sowing location (S) | 9·9 (2)** | 26·0 (2)*** | 4·6 (2) | 3·2 (2) | 33·5 (2)*** |
| Origin (O) | 323·7 (1)*** | 327·4 (5)*** | 107·3 (3)*** | 1·1 (1) | 223·3 (1)*** |
| O × S | 2004·2 (1)*** | 564·6 (10)*** | — | 325·1 (2)*** | 723·8 (2)*** |
| O × Y | 778·1 (1)*** | 395·7 (5)*** | 740·9 (3)*** | 186·3 (1)*** | 0·4 (1)*** |
| S × Y | 742·7 (1)*** | 4311·8 (2)*** | 1670·4 (2)*** | 3104·5 (2)*** | 1605·3 (1)*** |
P ≤ 0·01;
P ≤ 0·001.
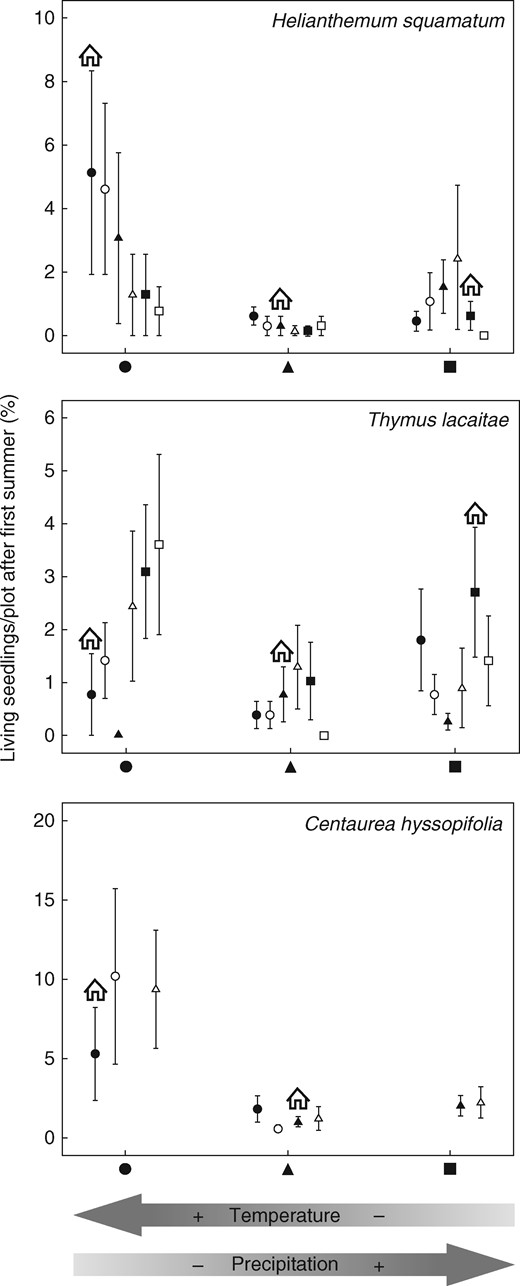
Percentage of surviving seedlings per plot (mean ± s.e.) at the three sowing locations (black circles, triangles and squares represent the three sowing locations and open circles, triangles and squares represent matched locations). Seeds sown at their home location are indicated by the house symbol. Better performance by seeds at their home site is indicative of local adaptation, while differences in the performance of seeds from a location along the climatic gradient are indicative of population phenotypic plasticity. The arrows at the bottom of the figure indicate the position of each location on the climatic gradient.
At the end of the study period, total seedling survival was <10 % in all species (Table 2). In terms of seedling lifespan, we detected a clear effect of year and its interaction with the sites of origin and sowing sites (Table 6). We also determined significant effects of the sowing and original sites on seedling lifespan. Helianthemum squamatum seedlings lived for longer at the most arid site and the effect of this sowing site was related directly to the aridness of the site where the seeds originated. By contrast, the lifespans of the remaining species were longer in the coldest and wettest sites (Fig. 5). The effect of the site of origin was generally quite local for H. squamatum and T. lacaitae, and especially for C. hyssopifolia. For the two narrowly distributed species, seedlings lived longer at their original sites.
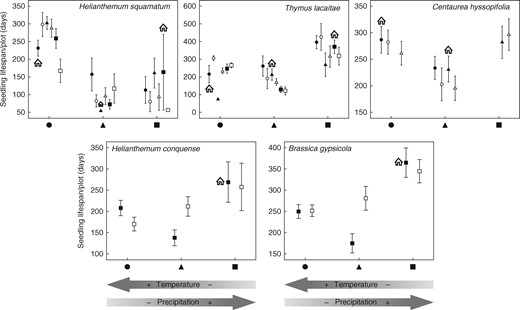
Seedling lifespan for each plot (mean ± s.e.) at the three sowing locations (black circles, triangles and squares represent the three sowing locations and open circles, triangles and squares represent matched locations). Seeds sown at their home location are indicated by the house symbol. Better performance by seeds at their home site is indicative of local adaptation, while differences in the performance of seeds from a location along the climatic gradient are indicative of population phenotypic plasticity. The arrows at the bottom of the figure indicate the position of each location on the climatic gradient.
DISCUSSION
We found no evidence of local adaptation in the gypsophytes considered in this study, even though the surveyed climate gradient was long. Indeed, our results demonstrated the prevalence of plastic and species-specific responses to climatic variations along an aridity gradient. Some species performed better in drier conditions (H. squamatum), whereas others exhibited the opposite pattern, with improved performance at the easternmost and cooler edge (T. lacaitae). In both cases, the seeds and seedlings from one particular site of origin performed better compared with others, irrespective of the position on the climate gradient where they were sown. This variation in plant performance agreed with the local abundances of regionally dominant gypsophytes. However, the restricted distribution of narrowly distributed gypsophytes could not be explained by the early-life responses identified in this study.
Species responses along the aridity gradient
We found no evidence of local adaptation, but germination was clearly modulated by the climatic conditions at the sowing sites. These results support the existence of some environmental cues for germination, thereby agreeing with previous studies of ephemeral gypsum specialists (Sánchez et al., 2014) as well as other Mediterranean and desert annual plants (Petrů and Tielbörger, 2008; Gremer and Venable, 2014). Water availability was not limited during the germination stage, especially in the wet and cold areas of the gradient. However, low temperatures may be a potential limiting factor for seed germination at such cold locations, where minimum daily temperatures can drop to around 0 °C throughout the spring season (Ninyerola et al., 2005). These temperatures are clearly below the optimum germination temperature for most Mediterranean plants (Corral et al., 1990; Sánchez et al., 2014), including most Iberian gypsophytes (Escudero et al., 1997). It should be noted that this cue operated in a similar manner on species that were naturally restricted to the colder site (H. conquense and B. gypsicola). This suggests that germination is not a key process that controls their confinement to the easternmost gypsum outcrops of the Tagus valley.
The climate at the site of origin but not the soil (Supplementary Data Appendix S2) was also an important predictor of germination performance, particularly for the two species distributed along the entire gradient. However, germination by T. lacaitae was better using seeds derived from the cooler sites, whereas the opposite effect was observed in H. squamatum, which exhibited higher germination rates using the seeds from drier sites. These results agree with those obtained in previous studies, which also found this germination behaviour in H. squamatum (Escudero et al., 1999), as well as with the abundance of both species along the gradient. Thus, the abundance of H. squamatum increased to the drier edge of the gradient, whereas T. lacaitae was more abundant in gypsum habitats located in cooler and more humid locations (Rivas-Martínez and Costa, 1970; Ferrandis et al., 2005). In both cases, ‘good seeds’ that originated from the optimal niche conditions performed best along the entire gradient and not only at their site of origin, thereby indicating a lack of local adaptation. Further studies should be performed to determine the extent to which the observed higher fitness of seeds from certain locations is related to population size and/or higher genetic variability (Reed, 2005; Leimu et al., 2006). It should also be noted that the differences in germination between seeds from matched locations were sometimes as great as those observed between seeds from different positions along the aridity gradient. In some cases the discrepancies were site-dependent, whereas in others they were consistent among sowing sites. This result suggests that local meteorological events such as summer thunderstorms can produce sharp variations in water availability, thereby generating a fitness mosaic with important differences between very close sites (Olano et al., 2011).
The growth of H. squamatum seedlings was also enhanced in individuals that emerged from seeds collected from the drier (‘optimal’) site. The remaining species also exhibited slight site-of-origin effects, but there were no clear relationships along the aridity gradient. In the case of the two narrow endemic species, the growth of seedlings appeared to depend on the origin of seeds at a fine spatial scale, whereas the performance differed between matched locations in the same direction, as observed previously for seed germination. However, the seedlings did not exhibit greater growth at their original sites, which also implies that their growth does not limit the colonization of warmer gypsum areas outside their current distribution range.
Seedling survival was relatively high in all of the species after their first summer compared with the survival rates observed for other Mediterranean species (Rey and Alcántara, 2000; Sánchez and Peco, 2007), but our results agreed with those obtained in previous studies of seedling survival in gypsum specialists (Escudero et al., 1999, 2000; De la Cruz, 2008). This result is counterintuitive with respect to the assumed strong restrictions imposed on plant life by gypsum soils (Escudero et al., 2015). The surprising ability of H. squamatum to obtain water directly from gypsum crystals during the summer water shortage (Palacio et al., 2014) could explain its high seedling survival rate during extremely stressful summers. Nevertheless, first-year survival and seedling lifespan were never higher in the home ranges in any of the study species, and thus there was a clear absence of local adaptation in all of these species. Interannual and local variations in rain events appear to be more likely factors that affect seedling survivorship. Interannual variations in the amounts and distribution of rainfall are well-known characteristics of the Mediterranean climate, and previous studies have demonstrated the influence of rainfall on plant performance (Olano et al., 2011) and seedling recruitment (Luzuriaga et al., 2012).
Local adaptation versus phenotypic plasticity
It is assumed that the importance of local adaptation is inversely related to habitat connectivity and its effects on gene flow among populations (Galloway and Fenster, 2000), especially when species lack structures that allow long-range seed dispersal. Naturally, gypsum habitats have island-like patterns and our gypsophytes lacked efficient dispersal structures (Escudero et al., 2015), but none of our results supported the existence of local adaptation during the first life stages of the five studied species. However, a plastic response in the observed processes and species emerged repeatedly.
A factor that might limit the local adaptation of gypsum specialists could be the high interannual variability that is typical of these Mediterranean habitats, especially at fine scales (Olano et al., 2011; Eugenio et al., 2012). Thus, strong climate variations may impose opposing selection pressures, thereby making the directionality of adaptation highly complex (Hereford and Winn, 2008; Leimu and Fischer, 2008; Parmesan and Hanley, 2015). In addition, this environmental variability could have exerted positive selection pressure on more plastic genotypes to produce populations highly adaptable to changing conditions based on their ability to produce optimal phenotypes under a broad range of environmental situations (Lázaro-Nogal et al., 2015).
Moreover, living on restrictive soils might require highly specific adaptations (Escudero et al., 2015) and limit the potential for further adaptive shifts (Lavergne et al., 2010). This hypothesis is similar to that proposed by Anacker et al. (2011), who observed that subsequent diversification was lower than that observed in non-endemic lineages after a lineage became ecologically specialized to serpentine soils. The results obtained by Gugger et al. (2015) also support this idea; they showed that species with specific adaptations to high elevation were less capable of shifting their reproductive phenology and they appeared to be more genetically constrained. Thus, a lower adaptive potential relative to non-specialized species could be a general feature of species that evolve restrictions to special soils or to any other limiting environment (Bieger et al. 2012), although further studies are needed in order to confirm this trend.
Are soil specialists sensitive or resistant to predicted increases in aridity?
The observed lack of local adaptation in gypsophytes, at least during their early life stages, as well as their plastic responses to climatic variability, may allow these species to perform better under future climate change scenarios. Furthermore, if the predicted environmental change, i.e. climate warming, leads to an increase in the stress level that these soil specialists experience, then they could be well positioned to persist locally due to their stress-tolerant characteristics (Damschen et al., 2012; Harrison et al., 2015). In particular, this may apply to regionally dominant gypsophytes, which possess a large number of stress-tolerant traits to cope with harsh conditions (Meyer, 1986; Escudero et al., 2015). Thus, H. squamatum could be favoured by increasing aridity in the medium and upper areas of our gradient, where it could achieve relatively high levels of dominance in the community. However, we must be cautious because not all the gypsophytes, even regionally dominant species, were equally capable of thriving under more arid conditions. For example, although T. lacaitae is widely distributed on gypsum outcrops in Central Spain, its regeneration ability was limited under more arid conditions.
Narrowly distributed species require special consideration. These species are also adapted to harsh conditions but in a less specific manner (Palacio et al., 2014), and this lower level of specialization could be related to their higher evolutionary potential (Anacker et al., 2011) compared with genuine gypsum specialists. Our reciprocal sowing experiment demonstrated that these two species performed quite well outside their actual distribution and current climatic range. Thus, if narrow endemics perform well at the drier edge of the gradient, they should be able to perform well on the cooler side of the gradient if it becomes warmer. However, these plant specialists have a narrow distribution so they are particularly vulnerable to potential synergies between global change drivers such as predicted aridification as well as habitat loss and fragmentation (Matesanz et al., 2009). Furthermore, gypsum outcrops are much scarcer further east of the actual limit of the habitat (Escavy et al., 2012), which may increase the probability of local extinction.
A further consideration is the long history of human use (>2000 years) of the habitats that we studied. Gypsum outcrops are highly fragmented and every remnant has probably been ploughed in the past, as well as being heavily grazed and deforested. Therefore, these abiotically restrictive habitats have been subjected to intense disturbance, so the species that survive at present are likely to be highly tolerant of disturbances. In fact, their pioneer character has been identified in previous studies (Martínez-Duro et al., 2010). Thus, we can assume that these species have passed through various abiotic and biotic evolutionary filters so they may be resistant to diverse types of disturbance, although this does not necessarily imply resistance to increasing aridity. However, there is evidence to support this suggestion because some typical functional traits of Mediterranean plants have been observed to reduce the damage caused by herbivores as well as conferring tolerance of drought and freezing temperatures (Agrawal et al., 2004). As a consequence, gypsophytes and more general soil specialists in the Mediterranean Basin could be more resistant to future environmental changes compared with soil specialists from regions without a long history of anthropogenic disturbance (Eskelinen and Harrison, 2015; Harrison et al., 2015).
Conclusions
According to our results, we do not expect that local adaptation will be a major process that might allow gypsophytes to cope with increasing aridity. Long-distance dispersal is also unlikely, so their stress-tolerant characteristics and observed ability to respond plastically to contrasting climatic conditions may be the only features that will allow gypsophytes to cope with increasing aridity (Bellard et al., 2012). However, higher stress levels will negatively affect germination, seedling establishment, and survival, particularly among species that perform better in milder conditions and those with restricted distributions. Furthermore, the observed species-specific response to different climatic scenarios could lead to important shifts in the local abundance and distribution of species under the increasing aridity that is predicted for these habitats.
SUPPLEMENTARY DATA
Supplementary data are available online at https://dbpia.nl.go.kr/aob and consist of the following. Appendix S1: arrangement of reciprocal sowing plots in the field and spatial distribution of the sowing points in each plot. Circles and crosses represent sowing points for 2007 and 2008 seeds, respectively. Appendix S2: soil properties (mean ± s.e.) at the three sowing sites (circles, Aranjuez; triangles, Mazuecos; squares, Torralba). Letters indicate significant differences between locations (post hoc Tukey test).
ACKNOWLEDGEMENTS
We thank Sonia García Rabasa, Raúl García Camacho and Eduardo Barahona Medina for their help with seed planting and Silvia Matesanz García and anonymous referees for their helpful comments on previous versions of the manuscript. This study was supported by the Spanish Science and Technology Commission (Roots) (CGL2015) and the Regional Government of Madrid (Remedinal 3-CM: S2013/MAE-2719).
LITERATURE CITED



