-
PDF
- Split View
-
Views
-
Cite
Cite
Abdoul Salam Koroney, Carole Plasson, Barbara Pawlak, Ramatou Sidikou, Azeddine Driouich, Laurence Menu-Bouaouiche, Maïté Vicré-Gibouin, Root exudate of Solanum tuberosum is enriched in galactose-containing molecules and impacts the growth of Pectobacterium atrosepticum, Annals of Botany, Volume 118, Issue 4, October 2016, Pages 797–808, https://doi.org/10.1093/aob/mcw128
Close - Share Icon Share
Abstract
Background and aims Potato (Solanum tuberosum) is an important food crop and is grown worldwide. It is, however, significantly sensitive to a number of soil-borne pathogens that affect roots and tubers, causing considerable economic losses. So far, most research on potato has been dedicated to tubers and hence little attention has been paid to root structure and function.
Methods In the present study we characterized root border cells using histochemical staining, immunofluorescence labelling of cell wall polysaccharides epitopes and observation using laser confocal microscopy. The monosaccharide composition of the secreted exudates was determined by gas chromatography of trimethylsilyl methylglycoside derivatives. The effects of root exudates and secreted arabinogalactan proteins on bacterial growth were investigated using in vitro bioassays.
Key Results Root exudate from S. tuberosum was highly enriched in galactose-containing molecules including arabinogalactan proteins as major components. Treatment of the root with an elicitor derived from Pectobacterium atrosepticum, a soil-borne pathogen of potato, altered the composition of the exudates and arabinogalactan proteins. We found that the growth of the bacterium in vitro was differentially affected by exudates from elicited and non-elicited roots (i.e. inhibition versus stimulation).
Conclusions Taken together, these findings indicate that galactose-containing polymers of potato root exudates play a central role in root–microbe interactions.
INTRODUCTION
Potato (Solanum tuberosum) belongs to the Solanaceae family, which comprises more than 2000 species, including economically important crops such as tomato (Solanum lycopersicum) and tobacco (Nicotianatabacum). Potato is cultivated for its tubers and represents one of the major food crops in the world, being surpassed only by rice (Oryza sativa), wheat (Triticum aestivum) and maize (Zea mays) in terms of both cultivated area and total crop production (King and Slavin, 2013). Worldwide crop production exceeds 320 million tons, representing human consumption for more than a billion people across South America, Europe, Asia and Africa (Haverkort, 1990). Potato is considered a promising crop to combat starvation worldwide and its cultivation is highly promoted by the United Nations (Diallo et al., 2011). Unfortunately, potato is subjected to many devastating diseases caused by soil-borne pathogens, which are responsible for considerable economic losses. For instance, the Pectobacterium species that is widely known under the name of Erwinia carotovora is a major threat for potato plants. Pectobacterium atrosepticum (formerly E. carotovora subsp. atroseptica) is a pathogen causing soft rot disease in both field conditions and during tuber storage (Latour et al., 2008; Yaganza et al., 2014). Pectobacteriumatrosepticum is responsible for primary infections facilitating subsequent attacks by other pathogens, such as those of the genus Dickeya, leading to blackleg disease and tuber soft rot (Pérombelon and Kelman, 1980; Priou and Jouan, 1996; Pérombelon, 2002). There is currently no effective method available to control the disease caused by P. atrosepticum. No chemical agents are efficient against these pathogens and cultural practices and storage conditions are unable to prevent the spread of the disease (Priou and Jouan, 1996; Czajkowski et al., 2011; Yaganza et al., 2014).
Previous research has shown that potato the rhizosphere is enriched in plant growth-promoting rhizobacteria and mycorrhizal fungi, two microorganism types that are currently used as biocontrol agents (Diallo et al., 2011). However, although a large number of studies have focused on tuber tissue infection, the role of root cells in the interaction with microbes remains unexplored (Horn et al., 2014). In order to develop new strategies for the protection of potato against root diseases it is necessary to gain a better understanding of the mechanisms controlling the interaction of the potato root with microorganisms and the response of root cells to pathogen attacks.
The root system plays an important role in shaping soil-borne microbe communities by secreting a wide range of compounds that stimulate and/or inhibit microorganism proliferation, thereby contributing to the maintenance of whole plant health (Wu and Van Etten, 2004; Berendsen et al., 2012). It is now recognized that the mechanisms involved in plant defence responses are organ-specific (Balmer et al., 2013). Although less well studied, a higher defensive state seems to occur in roots compared with above-ground organs of the plants (Attard et al., 2010; Millet et al., 2010; Balmer et al., 2013). In addition, the root immune response seems to vary between different root tissues (Millet et al., 2010; Cannesan et al., 2011, 2012). Several studies have shown that root caps display enhanced local resistance to pathogen attacks compared with the elongation zone, which is often described as the primary site of infection (Gunawardena and Hawes, 2002; Gunawardena et al., 2005; Cannesan et al., 2011). Protection of the root tip against abiotic and biotic stress has been shown to be due to the activity of border cells in many species, including pea (Pisum sativum), soybean (Glycine max) and rice (Oryza sativa) (Gunawardena and Hawes, 2002; Cannesan et al., 2011; Cai et al., 2013). Root border cells are known to originate from the root cap meristem, to detach individually and be released within the external environment (Hawes et al., 2003, 2012). All plant species produce populations of border cells that vary in number, shape, size and activity (Hawes et al., 2003; Driouich et al., 2007, 2012; Endo et al., 2011; Plancot et al., 2013). Border cells synthesize and secrete a large variety of substances, among which are many defence-related molecules that contribute to root protection (Wen et al., 2007, 2009; Cannesan et al., 2011; Plancot et al., 2013; Driouich et al., 2013). These molecules also contribute to the composition of the mucilage that often encloses border cells and influences microbial dynamic and activity within the rhizosphere.
The root exudate varies between species and depends on plant development and various external factors. The whole rhizodeposition secreted by roots into the soil is referred to as root exudate (Walker et al., 2003a). Root exudates comprise low-molecular-weight compounds such as amino acids, organic acids and phenolics as well as high-molecular-weight compounds, mainly present in the mucilage (Walker et al., 2003b; Liu et al., 2014). The term ‘mucilage’ refers to the slimy ‘halo’ that is visible at the microscopic level surrounding the root tip (Wen et al., 2007; York et al., 2016). This mucilage is mainly produced by root cap cells. It contains high amounts of polysaccharides, which confer sticky and gel-like properties (Durand et al., 2009). In addition, the mucilage also contains many low-molecular-weight molecules, proteins and extracellular DNA (Wen et al., 2007, 2009; Cannesan et al., 2012; York et al., 2016). Furthermore, by analogy with the ‘neutrophil extracellular trap’ (NET) described in mammalian blood cells, we have recently proposed that mucilage would constitute a ‘root extracellular trap’ (RET), involved in the protection of the root against soil-borne pathogens (Driouich et al., 2013). Within this mucilaginous matrix, different polymers would function in synergy with antimicrobial compounds and extracellular DNA to trap, neutralize and kill the pathogens.
The mucilage material is enriched in carbohydrates, with the presence of galactose, glucose, arabinose, fucose and xylose residues, which are components of cell wall polymers. Small amounts of galacturonic acid and glucuronic acid residues are also present in some species, such as pea and wheat (Knee et al., 2001). Based on the glycosidic linkage patterns established from exudates of different plants, it appears that polysaccharides are highly diverse and often unique within a plant species. Moreover, the nature of the polysaccharides and other glycan-containing molecules of root exudates plays a major role in mediating root–microbe interactions (Hinch and Clarke, 1980; Irving and Grant, 1984; Ray and Callow, 1988; Cannesan et al., 2012, Nguema-Ona et al., 2013). Although hydroxyproline has been reported to be a minor component in maize and cress exudates (Ray and Callow, 1988), glycoproteins such as arabinogalactan proteins (AGPs), which belong to the hydroxyproline-rich glycoprotein (HRGP) family, have been found to occur in root secretions of many plant species, such as pea, arabidopsis and Brassica napus (Knee et al., 2001; Durand et al., 2009; Cannesan et al., 2012).
In this paper we present the first study that characterizes border cells and exudates from root tips of S. tuberosum using a combination of microscopic and biochemical techniques. Our data demonstrate that root exudate contains unusually high amounts of galactose residues, suggesting that galactan-containing polymers such as AGPs are major components. In addition, we show that the composition of AGPs is altered in response to elicitors derived from the pathogenic bacterium Pectobacteriumatrosepticum. Finally, root exudates are shown to interfere with the growth and proliferation of the bacterium.
MATERIALS AND METHODS
Plant material
Tubers and in vitro plants of potato (Solanumtuberosum cultivar ‘Desiree’) were kindly provided by the Comité Nord Plants de Pommes de Terre, SIPRE, Achicourt, France.
Potato germs on potato tubers were cut and surface-sterilized in 70 % ethanol and in sodium hypochloride diluted 1/3 (v/v). They were then sown onto Murashige and Skoog medium containing 3·5 % (w/v) Bacto Agar supplemented with 3 % (w/v) sucrose (Durand et al., 2009). Plants were grown in a culture chamber under continuous light (120 mE m−2 s−1) for 18 h at 24 °C and 6 h for 18 °C. To prevent the roots penetrating the agar and the subsequent loss of border cells, plants were grown in vertically oriented Petri dishes. Microscopic observations of root tips were made on 10-d-old seedlings.
Collection of root exudates
Plants were grown in 18-h/6-h day/night cycles in 50 mL of culture medium without Bacto Agar. After 3 d, in vitro plants were washed (three times) in distilled water and transferred into distilled water for 3 d before collecting root exudates (10 plants per 50 mL in a Falcon tube; adapted from Chaparro et al., 2013). Root exudates were centrifuged (2500 g for 5 min) to remove cell debris and border cells, freeze-dried and weighed before use. The absence of remnant cells was checked using a bright-field microscope.
Histochemical staining and light microscopy
Roots were mounted on microscope slides in a drop of water for observations using bright-field microscopy. Ruthenium red dye (Sigma) was used at 0·05 % (w/v) in deionized water for 15 min. Roots were carefully washed in deionized water and observed using a bright-field microscope (Durand et al., 2009). To test viability, staining with 5 mm calcein acetoxymethyl ester (calcein-AM; Sigma) was performed as described in Vicré et al. (2005). Briefly, roots and root border cells were stained for 1 h, carefully washed in deonized water and observed using a microscope equipped with UV fluorescence (excitation filter, 490 nm; emission filter, 520 nm). Images were acquired with a Leica DCF 300FX camera.
Staining of callose was performed using aniline blue (Sigma-Aldrich) at a concentration of 1 mg mL−1 as described in Plancot et al. (2013).
Immunofluorescence localization of cell wall polysaccharide epitopes
The monoclonal antibodies (mAbs) specific for cell wall polysaccharides used in this study were JIM13 (Yates et al., 1996), LM1 (Smallwood et al., 1995) and LM2 (Smallwood et al., 1996). Roots from 10-d-old germs were fixed for 60 min in 1 % glutaraldehyde, 4 % paraformaldehyde in 50 mm 1,4-piperazinediethanesulfonic acid (PIPES) and 1 mm CaCl2, pH 7, and immunolabelled according to Willats et al. (2001). Roots were washed in phosphate-buffered saline (PBS) containing 1 % (w/v) bovine serum albumin (BSA) and then incubated overnight in JIM13 (1:50), LM1 (1:50) or LM2 (1:50), diluted in PBS–1 % BSA containing 1:30 normal goat serum, as described previously by Vicré et al. (2005). Roots were carefully washed and incubated with anti-rat immunoglobulin G (IgG) (dilution 1:5000) coupled to fluorescein isothiocyanate (Sigma). After washing in PBS–1 % BSA, roots were mounted in antifading agent (Citifluor; Agar Scientific) and examined using laser confocal microscope. Control experiments, in which the primary antiserum was omitted, were performed as described in Plancot et al. (2013).
Analysis of monosaccharide composition of root exudates
The sugar composition of root exudates was determined by gas chromatography of trimethylsilyl methylglycoside derivatives according to York et al. (1985) and using inositol as an internal standard. Briefly, samples were hydrolysed in 2 m trifluoroacetic acid for 2 h at 110 °C and then heated in dry 1 m HCl in methanol at 80 °C for 24 h for methanolysis. After evaporation of the methanol, the methyl glycosides were then converted into their trimethylsilyl derivatives by heating the samples for 30 min at 80 °C in hexamethyl disilizane:trimethyl chlorosilane:pyridine (3:1:9). After evaporation of the reagent, the samples were suspended in cyclohexane before being injected on a DB-1 column (Supelco) as described by Nguema-Ona et al. (2006). A temperature programme optimized for separation of the most common cell-wall monosaccharides (arabinose [Ara], fucose [Fuc], galactose [Gal], galacturonic acid [GalA], glucose [Glc], glucuronic acid [GlcA], mannose [Man], rhamnose [Rha], xylose [Xyl]) was used. Chromatographic data were integrated with GC Star Workstation software (Varian), each surface being corrected according to its response factor. All experiments were performed in triplicate and independently replicated six times with n =45 plants.
Methylation linkage analysis of S. tuberosum root exudates
Root exudates of S. tuberosum were methylated by the NaOH method of Ciucanu and Kerek (1984) (see also Nguema-Ona et al., 2012). Freeze-dried fractions (1–2 mg) were solubilized in 500 µL of DMSO, stirred overnight and sonicated prior to the start of methylation. Two or three NaOH pellets were ground and two tiny spatulas were added to a borosilicate glass test tube, and the contents were vigorously mixed. Two 25-µL portions of methyl iodide were added 20 min apart, followed after 20 min by a third addition of 50 µL, and the mixture was stirred for 2 h. The reaction mixture was quenched with 1 mL of 100 mg mL−1 freshly prepared sodium thiosulphate. One millilitre of dichloromethane was immediately added to the test tube and the contents of the tube were vigorously mixed to form an emulsion. After a brief centrifugation (30 s at 640 g), the upper aqueous phase was removed and the lower phase washed three times with water before drying under a stream of nitrogen. This first methylation step was repeated once. The methylated polysaccharides obtained were hydrolysed at 110 °C for 90 min in 0·5 mL of 2 m trifluoroacetic acid and the hydrolysed samples were dried under airflow and re-suspended in 100 µL of propan-2-ol before re-drying. The partially methylated monosaccharides were dissolved in 100 µL of 1 m NH4OH, and 500 µL of 20 mg mL−1 NaBD4 in DMSO was added to the mixture. This reduction reaction was performed overnight at room temperature. Excess reductant was destroyed with 100 µL of glacial acetic acid. One hundred microlitres of 1-methyl imidazole and 0·5 mL of acetic anhydride were successively added to the mixture. Acetylation of the partially methylated alditols was performed at room temperature for 15 min. Unreacted anhydride was destroyed with 1·5 mL of H2O, and the partially methylated alditol acetate (PMAA) derivatives were partitioned into 1 mL of dichloromethane, washed five times with water and dried. Finally, the PMAA derivatives were dissolved in 200 µL of dichloromethane. Derivatives were then injected into a GC-EI mass spectrometer composed of a Hewlett-Packard 6890 series gas chromatograph coupled with an Autospec mass spectrometer of EBE geometry (Micromass, Manchester, UK) equipped with an Opus 3.1 data system. Chromatographic separations were obtained using a Zebron Z5-MSi (30 m, 0·25 mm internal diameter, 0·25 mm film thickness; Phenomenex) capillary column. Helium was the carrier gas and the flow rate was 0·8 mL min−1. The temperature programme started at 100 °C for 1 min, ramped to 160 °C at 10 °C min−1 then to 220 °C at 2 °C min−1, and finally to 270 °C at 15 °C min−1 (maintained at 270 °C for 1 min). The temperature of the injector, the interface and the lines was 250 °C. Injections of 0·5 µL of samples were performed in splitless mode. Identification of the PMAA derivatives and deduction of their glycosidic linkages was achieved using a combination of reference data available publicly at http://www.ccrc.uga.edu/specdb/ms/pmaa/pframe.html, and from published PMAA spectra (Carpita and Shea, 1989). The linkage analysis experiments were performed in triplicate and independently replicated three times.
Immunoblots and electrophoresis
For SDS–PAGE analysis, total proteins were separated on 8 % polyacrylamide minigels (Bio-Rad) and blotted as described previously (Willats and Knox, 1996). For blot detection of arabinogalactan proteins and extensin epitopes, 15 µg of root exudate proteins was blotted on nitrocellulose membranes (Whatman; Protran BA 83 nitrocellulose, 0·2 mm) and blocked in Tris-buffered saline (TBS; 20 mm Tris, 500 mm NaCl, pH 7·5) containing 1 % Tween 20 (TBST). Exudates were incubated with primary antibody (1:25) in TBST for 2 h. After four washes with TBS (8 min per wash), blots were incubated for 2 h with anti-rat IgG peroxidase conjugate (Sigma) diluted 1:5000 in TBST. After four washes with TBST followed by one wash with TBS, enzyme activity was visualized with 30 % hydrogen peroxide (diluted in TBS) and methanol 4-chloronaphthol horseradish peroxidase colour reagent. The experiments were performed in triplicate and independently replicated three times with n =10 plants.
Dot blot analysis of root exudates was performed with a PR 648 Slot Blot Manifold, 48-well (GE Healthcare). One hundred microlitres of root exudate was blotted on nitrocellulose membranes (Whatman; Protran BA 83 nitrocellulose, 0·2 mm). After blocking in ·TBST (composition as stated above), the rest of the experiment was done as described above. The primary antibodies used were JIM13, JIM7, LM2, LM1, LM6, LM5 and INRA-RU1. All the antibodies were obtained from Plant Probes except INRA-RU1, which was kindly provided by F. Guillon (INRA, UR1268, France).
For the radial diffusion assay, 1 % (w/v) agarose gel containing 0·15 m NaCl, 0·02 % (w/v) NaNO3 and 10 µg mL−1 β-GlcY reagent was prepared according to Van Holst and Clarke (1986). The wells were filled with root exudates and incubated overnight at room temperature. Gum arabic from acacia (Fisher Scientific) was used as a standard. Determination of AGP presence was performed as described by Van Holst and Clarke (1986).
In vitro infection of S. tuberosum root with Pectobacterium atrosepticum
Pectobacterium atrosepticum strain CFPB 6276 was provided by Dr Xavier Latour (LMSM EA 4312, Université de Rouen, France). Pectobacteriumatrosepticum was grown in polygalacturonic acid (PGA) minimal medium supplemented with 0·4 % (w/v) polygalacturonic acid (Sigma-Aldrich, St Louis, MO) at 25 °C as previously described (Smadja et al., 2004a, b; Barbey et al., 2013). Infection assays were performed by deposition of 50 µL of a bacterial solution (15·107 Colony Forming Unit (CFU) mL−1) onto the root apex of S. tuberosum. After 5 d of infection, bacterial viability was assayed by adding one drop of the LIVE/DEAD Baclight Bacterial Viability Kit (Molecular Probes) at the location of the infection, following the instructions provided by the manufacturer. Observations were then made with a confocal laser microscope at the PRIMACEN imaging platform. Control experiments were carried out by using sterile water instead of bacterial suspension.
Pectobacterium atrosepticum growth curves
A culture of P. atrosepticum was grown in PGA minimal medium or in PGA minimal medium supplemented with root exudates (1 mg mL−1) from either non-elicited or elicited roots. Elicitation was performed using a culture filtrate of P.atrosepticum that was sterilized and freeze-dried. The elicitor preparation derived from P. atrosepticum was kindly provided by Dr F. Val (INRA Rennes, France) and was used for root elicitation at concentrations of 1 and 2 mg mL−1 (F. Val, pers. comm.). In order to determine whether AGPs are involved in P. atrosepticum growth, 5 µg mL−1 β-GlcY reagent was added to the growth medium. The growth of P. atrosepticum (25 °C) was determined by spectrophotometric measurements of OD580 conducted every 60 min for 16 h (adapted from Joshi et al., 2015). We determined that OD 0·1 corresponds to 1·107 CFU mL−1. Three biological replicates were performed, and each biological replicate consisted of three technical replicates.
Statistical analyses
Statistical significance was calculated by using two-way analysis of variance and the Tukey test. Differences were considered to be significant at P < 0·05.
RESULTS
Characteristics of root border cells from S. tuberosum
So far, the root architecture of S. tuberosum has never been investigated. Here, the morphology and detachment pattern of root border cells were examined in 10-d-old seedlings of S. tuberosum (Fig. 1). Staining of root tips with ruthenium red, a dye used for visualizing acidic polymers, revealed the presence of thick mucilage, in which border cells were embedded, surrounding the root tip (Fig. 1A). Two subpopulations of morphologically distinct border cells were released from the root tip; these consisted of small/rounded border cells, which were usually observed close to the root cap, and more elongated cells, mostly found located along the elongation zone. These cells were termed ‘small border cells’ and ‘elongated border cells’, respectively (Fig. 1B, C). Interestingly, large granular structures were clearly seen within the cytoplasm of both cell subpopulations (Fig. 1C). These structures were shown to contain starch, as revealed by Lugol staining and were most likely to be starch granules (Supplementary Data Fig. S1). These granules, in such a large number and size, are rarely seen in border cells of other species studied so far. Finally, staining with the vital dye calcein-AM demonstrated that both populations of border cells remained viable after their detachment and separation from the root tip (Fig. 1D).

Histochemical characterization of S. tuberosum root border cells. (A) Micrograph of a root tip stained with ruthenium red showing abundant mucilage (M) surrounding the root tip. Border cells are also seen at the root tip. (B) Calcofluor staining of the root tip showing border cell phenotypes. Two subpopulations of border cells are distinguishable: elongated border cells (eBC) and small/round-shaped cells (sBC). (C) Bright-field micrograph of a population of border cells showing abundant starch granules within the cells. (D) Root border cells stained with the vital dye calcein-AM. Fluorescence is indicative of cell viability. BC, border cells; RC, root cap. Scale bars: (A, B) = 100 µm; (D, E) = 20 µm.
Characterization of root exudates from S. tuberosum
As shown in Fig. 1A, a prominent halo of mucilage was found surrounding the root tip and border cells. The exudates were collected and the monosaccharide composition was determined by gas chromatography. As shown in Fig. 2, Gal was the most abundant sugar residue found in the exudates, accounting for 68·7 ± 0·2 % of total sugar residues. This suggests that galactan-containing polymers such as AGPs are major components of root exudates from S. tuberosum. Glucuronic acid represented 22·5 ± 0·1 % of the total sugars, whereas only very small amounts of GalA and neutral sugars (Ara, Rha, Xyl, Man and Fuc) were detected. Although less abundant, monosaccharides such as Rha, Gal and GalA suggested the presence of pectins in the exudates. Therefore, to gain additional information on the occurrence of pectins, an immunodot-binding assay was performed with the anti-pectin mAbs LM19 and JIM7, both specific for homogalacturonan (HG) epitopes, and the mAbs INRA-Ru1 and LM5, specific for epitopes associated with rhamnogalacturonan I (RG-I). All epitopes carried by HG and RG-I were detected in the exudates, consistent with the monosaccharide composition and confirming the presence of pectins (Table 1). Therefore, RG-I containing β-1,4 galactan side chains and HG is likely to occur in the exudates secreted by the root tip and border cells of potato.
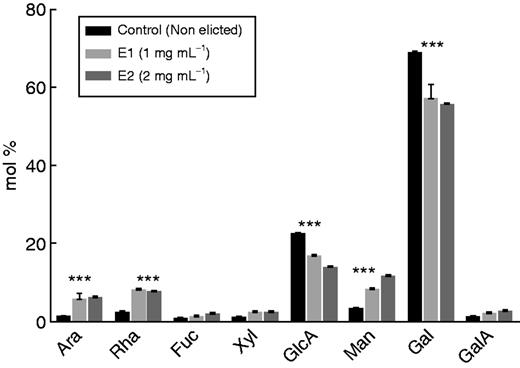
Monosaccharide composition (mol %) of S. tuberosum root exudates recovered from elicited and non-elicited (control) roots. Elicitation was done using an elicitor preparation from the bacterium P. atrosepticum. E1 and E2 correspond to the elicitor concentrations used (1 mg mL−1 for E1, 2 mg mL−1 for E2). Asterisks indicate a statistically significant difference (P<0·05).
Results of immunodot-binding assay performed on root exudates of S. tuberosum using different antibodies
| Antibody . | Reference . | Epitope . | Root exudates . |
|---|---|---|---|
| LM19 | Verhertbruggen et al. (2009) | Unesterified homogalacturonan | + |
| JIM7 | Willats et al. (2000) | Partially methyl-esterified epitope of homogalacturonan | + |
| INRA-Ru1 | Ralet et al. (2010) | [→2)-α-L-Rhap-(1→4)-α-D-GalpA-(1→] | + |
| LM10 | McCartney et al. (2005) | Unsubstituted and relatively low-substituted xylans | − |
| LM15 | Marcus et al. (2008) | XXXG xyloglucan motif | − |
| LM1 | Smallwood et al. (1995) | Extensin | + |
| LM5 | Jones et al. (1997) | β(1-4)-d-galactan | + |
| LM6 | Willats et al. (1998) | (1→5)-α-l-arabinan | + |
| JiM13 | Yates et al. (1996) | β-d-GlcA-(1→3)-α-d-GalA-(1→2)-α-l-Rha | + |
| LM2 | Smallwood et al. (1994) | β-linked GlcA | + |
| Antibody . | Reference . | Epitope . | Root exudates . |
|---|---|---|---|
| LM19 | Verhertbruggen et al. (2009) | Unesterified homogalacturonan | + |
| JIM7 | Willats et al. (2000) | Partially methyl-esterified epitope of homogalacturonan | + |
| INRA-Ru1 | Ralet et al. (2010) | [→2)-α-L-Rhap-(1→4)-α-D-GalpA-(1→] | + |
| LM10 | McCartney et al. (2005) | Unsubstituted and relatively low-substituted xylans | − |
| LM15 | Marcus et al. (2008) | XXXG xyloglucan motif | − |
| LM1 | Smallwood et al. (1995) | Extensin | + |
| LM5 | Jones et al. (1997) | β(1-4)-d-galactan | + |
| LM6 | Willats et al. (1998) | (1→5)-α-l-arabinan | + |
| JiM13 | Yates et al. (1996) | β-d-GlcA-(1→3)-α-d-GalA-(1→2)-α-l-Rha | + |
| LM2 | Smallwood et al. (1994) | β-linked GlcA | + |
+ and − refer to positive and negative labelling with the antibody, respectively
Results of immunodot-binding assay performed on root exudates of S. tuberosum using different antibodies
| Antibody . | Reference . | Epitope . | Root exudates . |
|---|---|---|---|
| LM19 | Verhertbruggen et al. (2009) | Unesterified homogalacturonan | + |
| JIM7 | Willats et al. (2000) | Partially methyl-esterified epitope of homogalacturonan | + |
| INRA-Ru1 | Ralet et al. (2010) | [→2)-α-L-Rhap-(1→4)-α-D-GalpA-(1→] | + |
| LM10 | McCartney et al. (2005) | Unsubstituted and relatively low-substituted xylans | − |
| LM15 | Marcus et al. (2008) | XXXG xyloglucan motif | − |
| LM1 | Smallwood et al. (1995) | Extensin | + |
| LM5 | Jones et al. (1997) | β(1-4)-d-galactan | + |
| LM6 | Willats et al. (1998) | (1→5)-α-l-arabinan | + |
| JiM13 | Yates et al. (1996) | β-d-GlcA-(1→3)-α-d-GalA-(1→2)-α-l-Rha | + |
| LM2 | Smallwood et al. (1994) | β-linked GlcA | + |
| Antibody . | Reference . | Epitope . | Root exudates . |
|---|---|---|---|
| LM19 | Verhertbruggen et al. (2009) | Unesterified homogalacturonan | + |
| JIM7 | Willats et al. (2000) | Partially methyl-esterified epitope of homogalacturonan | + |
| INRA-Ru1 | Ralet et al. (2010) | [→2)-α-L-Rhap-(1→4)-α-D-GalpA-(1→] | + |
| LM10 | McCartney et al. (2005) | Unsubstituted and relatively low-substituted xylans | − |
| LM15 | Marcus et al. (2008) | XXXG xyloglucan motif | − |
| LM1 | Smallwood et al. (1995) | Extensin | + |
| LM5 | Jones et al. (1997) | β(1-4)-d-galactan | + |
| LM6 | Willats et al. (1998) | (1→5)-α-l-arabinan | + |
| JiM13 | Yates et al. (1996) | β-d-GlcA-(1→3)-α-d-GalA-(1→2)-α-l-Rha | + |
| LM2 | Smallwood et al. (1994) | β-linked GlcA | + |
+ and − refer to positive and negative labelling with the antibody, respectively
To check for the presence of AGPs, immunodot assays using the mAbs LM2 and JIM13, specific for AGP epitopes, were also performed on the same root exudates. The data revealed that both epitopes were quite strongly detected, suggesting that AGPs are abundantly present in the secreted exudates (Table 1). Furthermore, we performed linkage analysis using methanolysis to characterize the linkage nature of Gal residues found in the exudates. The results showed that β-1,3 Gal-, β-1,6 Gal- and β-3,6 Gal residues were predominant, thus supporting the presence of AGPs as major components of the exudates (Table 2). Finally, along with AGPs, epitopes associated with extensin (another class of hydroxyproline-rich glycoproteins like AGPs) were also detected in the exudates using the mAb LM1. In contrast, xyloglucan epitopes could not be detected.
| Retention time (min) . | Methylation product . |
|---|---|
| 11·53 | T-Xylp |
| 16·48 | 3,5-Araf |
| 19·22 | 3-Galp |
| 20·47 | 6-Galp |
| 24·17 | 3,6-Galp |
| Retention time (min) . | Methylation product . |
|---|---|
| 11·53 | T-Xylp |
| 16·48 | 3,5-Araf |
| 19·22 | 3-Galp |
| 20·47 | 6-Galp |
| 24·17 | 3,6-Galp |
Linkages were deduced from electron-impact mass spectrometry of partially methylated alditol acetate derivatives.
| Retention time (min) . | Methylation product . |
|---|---|
| 11·53 | T-Xylp |
| 16·48 | 3,5-Araf |
| 19·22 | 3-Galp |
| 20·47 | 6-Galp |
| 24·17 | 3,6-Galp |
| Retention time (min) . | Methylation product . |
|---|---|
| 11·53 | T-Xylp |
| 16·48 | 3,5-Araf |
| 19·22 | 3-Galp |
| 20·47 | 6-Galp |
| 24·17 | 3,6-Galp |
Linkages were deduced from electron-impact mass spectrometry of partially methylated alditol acetate derivatives.
At the tissue level, immunofluorescence performed on root tips showed that AGP epitopes recognized by LM2 and JIM13 mAbs were abundant at the cell surface of root border cells and present in the mucilage (Fig. 3A, B). Control experiments performed by omitting the primary antibodies LM2 and JIM13 did not show any fluorescent staining (data not shown). We also investigated the occurrence of extensin, using the mAb LM1. Epitopes recognized by LM1were clearly found to occur at the cell surface of root border cells as well as in the mucilage, confirming the immunodot data (Fig. 3C, Table 1).
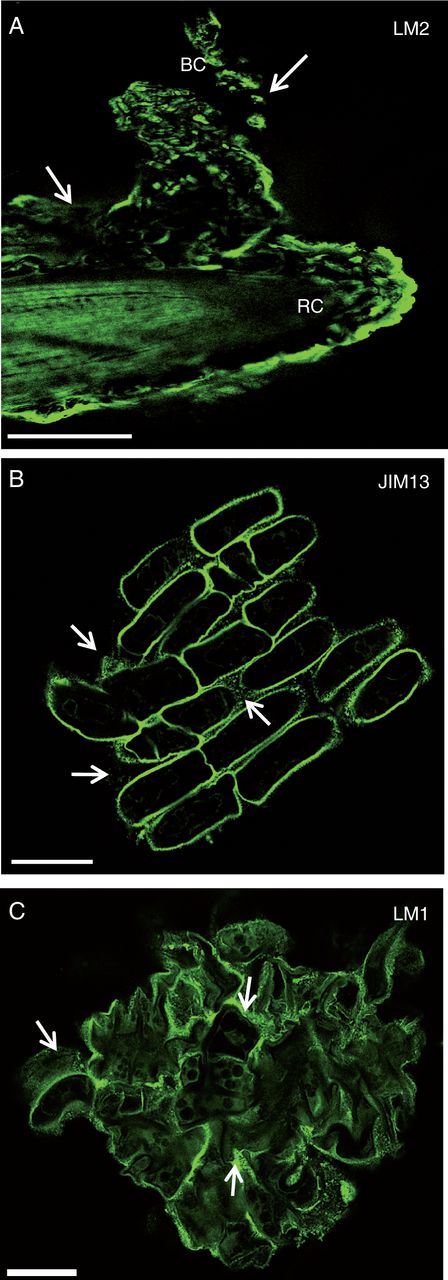
Immunofluorescence staining of root tips with the mAbs LM2 (A), JIM13 (B) and LM1 (C) recognizing AGP and extensin epitopes. Note the occurrence of the epitopes both at the root cell surface and within the mucilage (white arrows). RC, root cap; BC, border cells. Scale bars: (A) = 100 µm; (B, C) = 20 µm.
Effect of bacterial elicitation on monosaccharide composition of exudates
We investigated the composition of the exudates when root tips were treated with a culture filtrate from the soil-borne pathogen P. atrosepticum. To ascertain that the bacterial-derived elicitor induces a defence response in roots, we first examined the deposition of callose, a marker of defence, using aniline blue staining and microscopy. The data showed that callose deposition was clearly present in root tip and border cells when treated with the elicitor even at a concentration of 1 mg mL−1 (Supplementary Data Fig. S2). We then investigated the monosaccharide composition of the exudates after elicitation (3 d of treatment) of roots using two concentrations of elicitor (1 and 2 mg mL−1). A significant increase in Man residues was revealed in elicited samples compared with non-elicited controls (Fig. 2). However, control analysis showed that the P. atrosepticum elicitor preparation contained a significant amount of Man (data not shown), indicating that Man was not part of the plant response. A significant increase in Ara and Rha residues also occurred in response to elicitor treatment. Both monosaccharides were absent from the P. atrosepticum preparation (data not shown), suggesting that their increase is a significant part of the root tip response to elicitor. In contrast to Ara and Rha, a decrease in Gal and GlcA residues was observed in the presence of the elicitor compared with controls (Fig. 2). As a consequence, exudates from elicited plants exhibited a much higher Ara/Gal ratio (from 1:10 to 1:8 depending on the concentration of elicitors) than that found in the exudates of non-elicited roots (1:50). Such a change in sugar content could be related to modifications in AGP composition. Therefore, the quantity of AGPs in root exudates was estimated by radial gel diffusion and β-d-glucosyl Yariv staining. The β-D-glucosyl Yariv reagent was synthesized according to the protocol of Yariv et al. (1967). Grey spots are indicative of the presence of AGPs, the quantity being correlated to the diameter of the halo (Fig. 4). Staining with β-d-glucosyl Yariv revealed the presence of AGPs in root exudates from control and elicited samples. It also revealed an increase in AGP content after elicitor treatment, especially at the concentration of 2 mg mL−1.

Detection of arabinogalactan proteins in root exudates by gel diffusion assay with β-d-glucosyl Yariv reagent. Exudates were recovered from non-elicited control (C) and elicited (E1 and E2) roots. The halos correspond to the intensity of staining with the β-d-glucosyl Yariv reagent revealed by the gel diffusion assay. Gum arabic was used at different concentrations (1·25–50 µg mL−1) as a control and standard. Elicitation was applied using an elicitor preparation from the bacterium P. atrosepticum. E1 and E2 correspond to the elicitor concentrations used (1 mg mL−1 for E1, 2 mg mL−1 for E2).
To analyse further the impact of elicitation on AGP composition, exudates were separated by SDS–PAGE, blotted and probed with the anti-AGP mAbs JIM13, JIM15 and LM2. The data revealed that JIM13 bound to a smear of material of molecular weight between 34 and130 kDa in control as well as in elicited samples (Fig. 5A). In the case of LM2, a band of ∼34 kDa, not seen in control samples, strongly reacted with the antibody in elicited samples (Fig. 5B). The intensity of the signal was more pronounced at the concentration of 1 mg mL−1 of elicitor than at 2 mg mL−1. Anti-JIM15 showed a weak signal in non-elicited plants, revealing two bands faintly stained at 28 and 34 kDa (Fig. 5C). In elicited samples, the JIM15 antibody reacted very strongly with two bands at 25 and 40 kDa, not detected in control samples, with 1 and 2 mg mL−1 of elicitor, respectively. This further supports the idea that AGP composition/structure is modified in the exudates secreted by elicited roots.
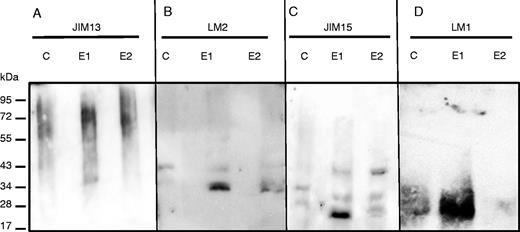
SDS–PAGE and western blot analysis of proteins from root exudates of non-elicited (C, control) and elicited (E1 and E2) plants. Proteins were run on gels, blotted and probed with antisera recognizing either arabinogalactan protein epitopes JIM13 (A), LM2 (B) and JIM15 (C) or extensin epitope LM1 (D). The epitopes recognized by each antibody are presented in Table 1. E1 and E2 correspond to the elicitor concentrations used (1 mg mL−1 for E1, 2 mg mL−1 for E2).
However, one should not rule out the possibility that differences in monosaccharide composition observed in elicited samples could also arise from modifications in extensin composition. Western blotting using the anti-extensin mAb JIM11 did not reveal any significant difference between samples from non-elicited and elicited roots and detected the presence of a smear ranging between 28 and 130 kDa (data not shown). In contrast, a major band ranging between 20 and 34 kDa strongly reacted with the mAb LM1 in exudates from plants treated with the elicitor at 1 mg mL−1 compared with other conditions (Fig. 5D). The smear was very weakly detected in samples elicited at 2 mg mL−1.
Effect of root exudates on the growth of P. atrosepticum
While P. atrosepticum is known to infect potato tubers, causing tuber blight diseases, root colonization and infection remain poorly studied. Here, we assessed the effect of root exudates on P. atrosepticum growth in vitro. First, we checked whether the bacteria bound to roots when these were inoculated with a solution of P. atrosepticum at a concentration of 15·107 CFU mL−1. As shown in Fig. 6, the bacteria were clearly seen over the surface of the root tip, border cells and exudates. We then monitored bacterial growth over 15 h by measuring the OD580 every 60 min in the presence of a standard growth medium or medium supplemented with root exudate (Fig. 7). Compared with bacteria cultured in the standard medium, bacterial biomass production in the presence of the exudate-containing medium increased significantly. As a control, we showed that root exudates were free of contaminating bacteria by measuring the OD580 every 60 min over 15 h in the absence of inoculation with P. atrosepticum (Fig. 7). AGPs were recently reported to be key components in root–microbe interactions (Nguema-Ona et al., 2014). Root exudates from potato are unusually enriched in Gal-containing polymers, including AGPs. To check whether AGPs present in root exudates could affect bacterial growth, exudates were incubated overnight with 5 µg mL−1 β-d-glucosyl Yariv reagent and added to the growth medium. The β-d-glucosyl Yariv reagent binds and precipitates AGPs, thus interfering with their function (Yariv et al., 1967; Sardar et al., 2006; Kitazawa et al., 2013; Marzec et al., 2015). When exudate treated with β-d-glucosyl Yariv reagent was added to the growth medium, biomass production of P. atrosepticum was only slightly reduced.
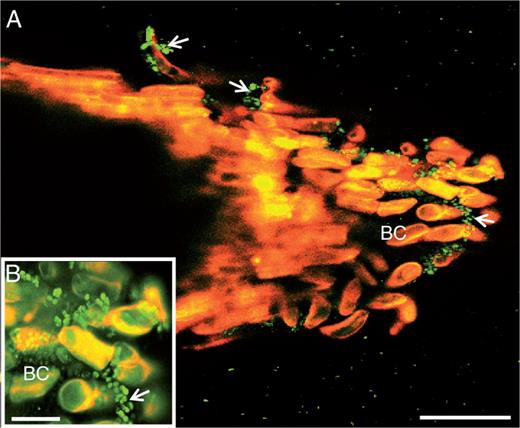
Laser confocal microscopy image showing binding of the bacterium P. atrosepticum to S. tuberosum root tip. Note the presence of bacteria over border cells (arrowheads and inset). Green spots correspond to bacteria. BC, border cells. Scale bars: (A) = 86 µm (A); (B) = 20 µm.
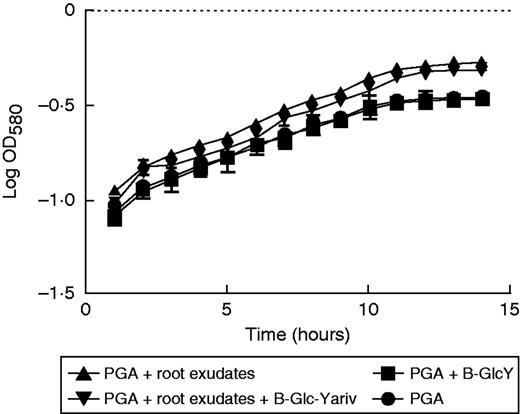
Effects of root exudate from S. tuberosum plants on the growth of P. atrosepticum. Graphic representation of bacterial growth curve in a standard growth medium (PGA) or medium supplemented with root exudate (1 mg mL−1). In addition, β-GlcY reagent (5 µg mL−1) was added to the growth medium in order to precipitate AGPs contained in the root exudates. Bars show means ± s.e. of three biological replicates; P< 0·05 is significant.
Finally, we tested the effect of different media supplemented with exudates collected from elicitor-treated plants on bacterial growth. Under these conditions biomass production of bacteria was slightly reduced when compared with that of bacteria grown in a medium containing exudate from non-elicited plants (Fig. 8). Addition of β-d-glucosyl Yariv reagent to exudate from elicited plants did not affect significantly the growth of P. atrosepticum bacteria.
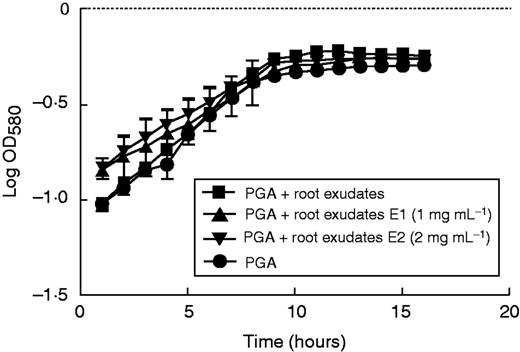
Effects of root exudate from elicited S. tuberosum plants on the growth of P. atrosepticum. Graphic representation of bacterial growth curve in a standard growth medium (PGA) or medium supplemented with root exudate (1 mg mL−1). E1, bacteria growth in medium supplemented with root exudate (1 mg mL−1) from elicited plants with 1 or 2 mg mL−1 (E1 and E2, respectively) elicitor derived from P. atrosepticum. Bars show means ± s.e. of three biological replicates; P<0·05 is significant.
DISCUSSION
In the context of the ever-increasing human population and water shortage, the potato crop is essential to the food security of hundreds of millions of people worldwide. Potato diseases such as common scab, tuber soft rot and powdery scab caused by soil-borne pathogens remain a major threat affecting tuber quality and yield (Thangavel et al., 2014). So far, most of the studies on S. tuberosum have been related to infection of tubers, leaving the root system unexplored. Considering the importance of root border cells and their exudates in shaping the soil microflora, it is thus essential to acquire knowledge of the potato root system and its function in plant defence against pathogens. A comprehensive understanding of the cellular and molecular mechanisms controlling interactions between roots, exudates and microorganisms can help in improving protection during tuber formation and growth.
The present study is one of the first reports that characterizes the potato root tip, border cells and exudate. Root tips of S. tuberosum released individual cells characteristic of the ‘classical’ root border cells embedded in thick mucilage, similar to those described in pea (Hawes et al., 2003). Based on their morphology, two subpopulations of root border cells, namely small and elongated border cells, were easily distinguishable. In pea, three subpopulations of border cells were previously identified, corresponding to small, intermediate and elongated border cells. In pea, however, only small and intermediate border cells were found to be living cells capable of developing defence responses against pathogens (Cannesan et al., 2011). In potato, elongated border cells were also stained with calcein-AM, indicating that, unlike in pea, both subpopulations are viable cells that might function in root defence. Also unlike in pea, potato border cells were found to be highly enriched in large starch granules. It was speculated that starch provides an energy and carbon source for root border cells of Medicago truncatula (Watson et al., 2015). It is likely that starch also plays an important role in the survival of potato root border cells after their release within the rhizosphere.
Our data also show the occurrence of a thick mucilage that surrounds border cells. In plants, root mucilage is mainly produced and secreted by the root cap and border cells (Moody et al., 1988; Vicré et al., 2005; Ma et al., 2010). Carbohydrates have been shown to be major components of root mucilage in many plant species, including cowpea, pea, wheat and maize (Moody et al., 1988; Cannesan et al., 2011). They are also important for the interaction of root with microbes (Hinch and Clarke, 1980; Longman and Callow, 1987). In the present study we show that potato root exudates contain a high amount of Gal (68·7 %), suggesting that galactan-containing polymers are major components of these secretions. Such a high content of Gal residues has never been reported in any other plant species studied so far; the Gal content was always found to be lower in root exudates, not exceeding 30–35 %. For instance, the Gal content was shown to be 33 % for maize, 28 % for cowpea, 20 % for rice and 16 % for wheat (Chaboud and Rougier, 1984; Moody et al., 1988). Although we show that galactan-containing polymers such as RGI are constituents of potato root exudates, AGPs appear to be the most abundant molecules. This observation is in agreement with previous findings reporting the presence of AGPs in quite high amounts in root exudates of various plant species (Moody et al., 1988; Durand et al., 2009; Ma et al., 2010; Cannesan et al., 2012). However, AGPs were not the only HRGPs represented in the root exudates of potato. Interestingly, LM1-recognized epitopes carried by extensin glycoproteins were also detected in the exudates. Whereas extensins are known to accumulate in plant cell walls, resulting from their structural reinforcement during biotic stress, their occurrence in root exudates has never been reported. The high content of Gal residues present in potato root exudates seems to be mostly associated with AGPs and to a lesser extent with extensin and RG-I. Recent data highlight the importance of HRGPs such as AGPs and extensin in plant–microbe interactions (Zhu et al., 2003; Xie et al., 2011; Nguema-Ona et al., 2012, 2014; Plancot et al., 2013). For instance, in vitro assays revealed that AGPs from pea root cap and border cells could interfere with the development of the pathogenic oomycete Aphanomyces euteiches (Cannesan et al., 2012). Such species-specific interaction is probably correlated to the high complexity and specificity of the AGP carbohydrate moiety. However, the molecular mechanisms controlling such interactions are still not fully elucidated. Interestingly, our western blot data show that treatment with elicitor preparations from P. atrosepticum induces modifications of AGPs and extensin in root exudates of S. tuberosum. Elicitation also causes an increase in labelling with the anti-extensin mAb LM1. Whereas no change in AGP labelling was detected in our conditions with the JIM13 antibody, the LM2 and JIM15 AGP signals strongly increased after treatment with the elicitor. This indicates that specific subpopulations of root AGPs might be involved in the response to elicitors and defence against pathogens. It is worth noting that an increase in the content of the AGPs recognized by the mAb LM2 was previously detected upon inoculation with pathogenic Fusarium oxysporum and in response to fusaric acid treatment in the roots of wax gourd (Xie et al., 2011). Modification of the anti-extensin LM1 pattern of labelling also occurs in response to elicitors in root border-like cells of Arabidopsisthaliana and flax (Plancot et al., 2013). These observations support a role for LM2, JIM15 and LM1 epitope-containing molecules in defence responses of root cells against pathogens.
Our data also show that root exudates from S. tuberosum stimulate the biomass production of P. atrosepticum. In order to check whether AGPs contained in the exudates interfere with the development of P. atrosepticum, root exudates were pre-incubated with β-d-glucosyl Yariv reagent before use. We previously reported that AGPs from pea root border cells were able to alter the development of A.euteiches (Cannesan et al., 2012). In the present study, AGPs had no effect on the growth of the bacterium P. atrosepticum. On the contrary, a slight decrease in bacterial growth was observed when the bacteria were incubated with β-d-glucosyl Yariv-treated exudates. It should be noted that it is difficult to estimate the proportion of AGPs present in root exudates that are effectively bound to β-d-glucosyl Yariv (Kitazawa et al., 2013). Furthermore, we cannot rule out the possibility that β-d-glucosyl Yariv-bound AGPs remained biologically active. As a consequence, the effects of AGPs on P. atrosepticum growth revealed in this study might very well be underestimated. It is well known that soil-borne microorganisms utilize root exudates for growth and multiplication (Bais et al., 2006). Although we cannot exclude the possibility that AGPs might be a source of nutrients for bacteria, AGPs or their carbohydrates could also act as signalling molecules that activate mechanisms controlling bacterial cell proliferation. However, it remains to be established whether AGPs affect bacterial binding and growth on potato root in vivo in a similar manner.
Together, our findings highlight the importance and complexity of galactan-containing molecules released by root cap and border cells into exudates in controlling plant root interactions with soil-borne microbes.
ACKNOWLEDGEMENTS
The authors thank Dr Xavier Lathour (LMSM, EA 4312, Evreux, Université de Rouen, France) and Dr Florence Val (Agrocampus Ouest Rennes, France) for providing P. atrosepticum strains and the elicitor preparation from P. atrosepticum, respectively. Special thanks are due to Dr Virginie Deveaux (Comité Nord, Achicourt, France) for providing tubers and vitroplants of potato and Dr Corinne Loutelier-Bourhis (COBRA UMR6014 and FR3038, Université de Rouen, France) for linkage analysis of root exudates using methanolysis. We also thank Dr Eric Nguema-Ona for helpful and constructive comments during the course of this work. This work was supported by La Région de Haute Normandie and le Grand Réseau de Recherche-Végétal, Agronomie, Sol et Innovation, l’Université de Rouen and Le Fonds Européen FEDER. Abdoul Koroney was funded by a PhD fellowship from the French embassy in Niger (Bourse de Cooperation Française).
LITERATURE CITED



