-
PDF
- Split View
-
Views
-
Cite
Cite
Tatsuya Maruhashi, Yoshihiko Kinoshita, Ryoji Ozono, Mitsuaki Nakamaru, Masanori Ninomiya, Jiro Oiwa, Takuji Kawagoe, Osamu Yoshida, Toshiyuki Matsumoto, Yasuo Fukunaga, Kotaro Sumii, Hironori Ueda, Nobuo Shiode, Kosuke Takahari, Yasuhiko Hayashi, Yujiro Ono, Yukiko Nakano, Masakazu Takahashi, Yasuki Kihara, Yukihito Higashi, Hiroshima NOCTURNE Research Group, Significant Correlates of Nocturnal Hypertension in Patients With Hypertension Who Are Treated With Antihypertensive Drugs, American Journal of Hypertension, Volume 36, Issue 6, June 2023, Pages 287–296, https://doi.org/10.1093/ajh/hpad014
Close - Share Icon Share
Abstract
Nocturnal hypertension assessed by a home blood pressure monitoring (HBPM) device is associated with an increased risk of cardiovascular events. However, it is still difficult to assess nighttime blood pressure (BP) frequently. The purpose of this cross-sectional study was to identify significant correlates of nocturnal hypertension assessed by an HBPM device in patients with hypertension who are treated with antihypertensive drugs.
We measured nighttime BP, morning BP, and evening BP by an HBPM device for 7 consecutive days in 365 medicated patients with hypertension.
Of the 365 subjects, 138 (37.8%) had nocturnal hypertension defined as a mean nighttime systolic BP of ≥ 120 mm Hg. Receiver operating characteristic curve analyses showed that the diagnostic accuracy of morning systolic BP for subjects with nocturnal hypertension was significantly superior to that of evening systolic BP (P = 0.04) and that of office systolic BP (P < 0.001). Multivariate analysis revealed that morning systolic BP of 125–<135 mm Hg (odds ratio [OR], 2.26; 95% confidence interval [CI], 1.13–4.58; P = 0.02), morning systolic BP of ≥ 135 mm Hg (OR, 16.4; 95% CI, 8.20–32.7; P < 0.001), and a history of cerebrovascular disease (OR, 3.99; 95% CI, 1.75–9.13; P = 0.001) were significantly associated with a higher risk of nocturnal hypertension and that bedtime dosing of antihypertensive drugs was significantly associated with a lower risk of nocturnal hypertension (OR, 0.56; 95% CI, 0.32–0.97; P = 0.04).
Morning systolic BP of ≥ 125 mm Hg, a history of cerebrovascular disease, and bedtime dosing were significant correlates of nocturnal hypertension in medicated patients with hypertension, and may help detect this risky BP condition.
University Hospital Medical Information Network Clinical Trials Registry (UMIN000019173).
In major hypertension guidelines, measurements of out-of-office blood pressure (BP) using ambulatory blood pressure monitoring (ABPM) or home blood pressure monitoring (HBPM) have been recommended for the diagnosis and treatment of hypertension since accumulating evidence has shown that out-of-office BP is a better predictor than office BP of cardiovascular events.1–6 Self-measured HBPM is strongly recommended due to its high reproducibility and prognostic value for cardiovascular events.7–9
ABPM has been historically used as the gold standard for the measurements of nighttime BP. However, ABPM devices are expensive and frequent cuff inflations during sleep may cause discomfort and sleep disturbance. Therefore, ABPM is not widely used in clinical practice. Recently, technological advancement has made it possible to measure nighttime BP by using an HBPM device equipped with a timer that triggers automated BP measurements during sleep. Recent studies have shown that nocturnal hypertension assessed by an HBPM device is significantly associated with an increased risk of cardiovascular events,10,11 suggesting that assessment of nighttime BP by an HBPM device to check for the presence or absence of nocturnal hypertension may provide additional information for more precise cardiovascular risk assessment.
Since an HBPM device developed for the measurement of nighttime BP is not so expensive and it is characterized by its low noise during BP measurement and is equipped with a long tube so that the device can be placed away from a subject during sleep, the assessment of nighttime BP by an HBPM device may be more feasible and acceptable than assessment by an ABPM device. However, it is still not convenient for patients to assess nighttime BP frequently using an HBPM device. Therefore, it is important to clarify which clinical variables are associated with nocturnal hypertension for predicting the presence or absence of nocturnal hypertension, especially in medicated patients with hypertension since the impact of nighttime systolic BP on cardiovascular events may be greater in patients receiving antihypertensive drugs than in patients not receiving antihypertensive drugs.12 In the Japan Morning Surge Home Blood Pressure (J-HOP) study, relationships between nighttime BP measured by an HBPM device and clinical variables were investigated in patients with one or more cardiovascular risk factors or cardiovascular disease.13 However, approximately 20% of the subjects in the J-HOP study were not receiving antihypertensive drugs. Therefore, there is little information on the associations between nocturnal hypertension assessed by an HBPM device and clinical variables in patients with hypertension who are treated with antihypertensive drugs. Moreover, there is little information on the prevalence of nocturnal hypertension in medicated patients with hypertension who have achieved the home systolic BP target of < 125 mm Hg currently recommended by the Japanese Society of Hypertension (JSH) guidelines for the management of hypertension published in 2019.2 We therefore measured nighttime BP by using an HBPM device to investigate the associations between nocturnal hypertension assessed by an HBPM device and clinical variables in patients with hypertension who were being treated with antihypertensive drugs.
Methods
Study design
This was a cross-sectional study. This study was conducted in patients from the Hiroshima Registry for Evaluation and Treatment of Nocturnal and Early Morning Hypertension (Hiroshima NOCTURNE). Hiroshima NOCTURNE is a prospective multicenter study to investigate whether elevated nighttime BP assessed by an HBPM device can be normalized by aggressive antihypertensive drug therapy and to evaluate the effect of reduction of nighttime BP on future cardiovascular events in patients with hypertension receiving antihypertensive drugs. Patients aged 20 years or more who had been treated with antihypertensive drugs for more than 3 months with a diagnosis of hypertension defined in the JSH guidelines for the management of hypertension were enrolled.14 Subjects with severe valvular heart disease, moderate to severe heart failure (NYHA class III or IV), or lethal arrhythmia with an implantable cardioverter defibrillator, subjects receiving dialysis for end-stage renal disease, subjects with malignant disease or hepatic cirrhosis, subjects receiving corticosteroid therapy or immunosuppressive therapy, possible pregnant women, pregnant women, and lactating women were excluded. Between October 2015 and March 2019, a total of 443 Japanese adults who were being treated with antihypertensive drugs were recruited from 6 hospitals and 9 affiliated clinics in Hiroshima Prefecture. Some of the data from Hiroshima NOCTURNE were previously reported elsewhere.15 Patients without measurements of morning BP or evening BP (n = 75) and patients without measurements of office BP (n = 3) were excluded. Finally, 365 patients (224 men and 141 women; mean age, 67.9 ± 10.3 years; age range, 33–89 years) were enrolled in this study. Diabetes was defined according to the American Diabetes Association recommendation.16 Dyslipidemia was defined according to the third report of the National Cholesterol Education Program.17 We defined smokers as those who had ever smoked. Estimated glomerular filtration rate (eGFR) was calculated using the Japanese equation.18 Chronic kidney disease (CKD) was defined as eGFR < 60 ml/min per 1.73 m2.19 Coronary heart disease (CHD) was defined as a history of myocardial infarction, angina pectoris, or unstable angina. Cerebrovascular disease included ischemic stroke, hemorrhagic stroke, and transient ischemic attack. Primary aldosteronism included aldosterone-producing adenoma and idiopathic hyperaldosteronism. The ethical committees of our institutions approved the study protocol. The study was executed in accordance with the Helsinki Declaration of 1975. Written informed consent for participation in the study was obtained from all participants. The protocol was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000019173).
Measurements of blood pressure
HBPM was performed using a cuff oscillometric device (HEM-7252G-HP or HEM7080IC; Omron Healthcare Co., Kyoto, Japan) that could be set to measure BP automatically during sleep (nighttime BP). All data, including data for BP, heart rate and measurement time, obtained by using HEM-7252G were transmitted automatically to the Medical LINK program, a cloud-based remote monitoring system, provided by Omron Healthcare. All data obtained by using HEM7080IC were stored in its memory. Patients were instructed to measure their morning BP twice, evening BP twice before going to bed, and nighttime BP during sleep for 7 consecutive days. Patients were advised to measure their morning BP within 1 hour after waking up, after urination, before breakfast and taking antihypertensive drugs, and after 1–2 min rest in a sitting position and to avoid smoking, alcohol, and caffeine consumption before measurement in accordance with the JSH guidelines for the management of hypertension in 2014.14 The average of all morning systolic BP measurements was used for analyses as morning systolic BP. The average of all evening systolic BP measurements was used for analyses as evening systolic BP. Nighttime BP was measured automatically at 2:00, 3:00, 4:00, and 5:00 AM (4 time points). In patients who got up before 5:00 AM, nighttime BP was measured at 1:00, 2:00, 3:00, and 4:00 AM. Nocturnal hypertension was defined as the average of all nighttime systolic BP measurements ≥ 120 mm Hg.2,20 The nocturnal dipping level was calculated as a percent change of nighttime systolic BP to daytime systolic BP defined as an average of morning systolic BP and evening systolic BP measurements: [(nighttime systolic BP − daytime systolic BP)/daytime systolic BP] × 100 (%). The nocturnal dipping pattern was defined according to the mean of daily nocturnal dipping level. A riser pattern was defined as mean nocturnal dipping level of ≥ 0% (nighttime systolic BP higher than daytime systolic BP).21 Office BP was measured in accordance with the JSH guidelines for the management of hypertension in 2014.14 In brief, BP was measured twice in a sitting position, and the mean value of the 2 measurements was used for analyses.
Statistical analysis
All reported Probability values were 2-sided, and a Probability value of < 0.05 was considered statistically significant. Continuous variables are summarized as means ± SD and were compared by using an unpaired Student t test. Categorical variables are presented as frequencies and percentages and were compared by means of the chi-square test. Univariate linear regression analyses were performed to assess relationships between nighttime systolic BP, morning systolic BP, evening systolic BP, and office systolic BP. Receiver operating characteristic (ROC) curve analyses were carried out to assess the sensitivity and specificity of morning systolic BP, evening systolic BP, and office systolic BP and to confirm the optimal cutoff value of morning systolic BP to diagnose subjects with nocturnal hypertension. Area under the curve (AUC) values were calculated and the differences in AUC values were compared using the method of Delong et al. with Bonferroni’s test for post hoc comparisons.22 The cutoff value was determined according to the highest Youden index from the ROC curve analysis. Univariate logistic regression analyses were performed to assess relationships between nocturnal hypertension, the riser pattern, and variables. Multiple logistic regression analyses were performed to identify significant variables associated with nocturnal hypertension and the riser pattern from the covariates with P < 0.10 in the univariate logistic regression analyses, with age ≥ 65 years and sex forced into the model. Among blood pressure parameters, morning systolic BP was entered into the model for the associations between nocturnal hypertension and variables because morning systolic BP had the strongest correlation with nighttime systolic BP in the univariate analysis. The data were processed using JMP version Pro 16.0 (SAS institute, Cary, NC).
Results
Baseline clinical characteristics
The baseline clinical characteristics are summarized in Table 1. Of the 365 subjects, 300 (83.3%) had dyslipidemia, 92 (25.5%) had diabetes mellitus, 102 (28.3%) had CKD, 53 (14.7%) had CHD, 38 (10.6%) had cerebrovascular disease, 34 (9.6%) had sleep apnea syndrome, 7 (1.9%) had primary aldosteronism, and 165 (47.8%) were smokers. All of the subjects were being treated with antihypertensive drugs: 83.0% were on calcium channel blockers, 72.9% were on angiotensin II receptor blockers, 3.5% were on angiotensin-converting enzyme inhibitors, 15.3% were on β-blockers, 1.9% were on α-blockers, 22.2% were on diuretics, and 5.2% were on mineralocorticoid receptor antagonists. One hundred forty-five subjects (39.7%) took antihypertensive drugs at bedtime. Mean values were 130.1 ± 15.9 mm Hg for office systolic BP, 131.0 ± 13.3 mm Hg for morning systolic BP, 125.3 ± 14.4 mm Hg for evening systolic BP, and 117.4 ± 12.7 mm Hg for nighttime systolic BP.
| . | All . | Nonnocturnal hypertension . | Nocturnal hypertension . | . |
|---|---|---|---|---|
| Variables . | (n = 365) . | (n = 227) . | (n = 138) . | P value . |
| Age, years | 67.9 ± 10.3 | 66.2 ± 10.8 | 70.5 ± 9.0 | <0.001 |
| Age ≥ 65 years | 262 (71.8) | 149 (65.6) | 113 (81.9) | <0.001 |
| Men, n (%) | 224 (61.4) | 138 (60.8) | 86 (62.3) | 0.77 |
| Body mass index, kg/m2 | 24.6 ± 3.6 | 24.7 ± 3.4 | 24.6 ± 3.8 | 0.89 |
| Body mass index ≥ 25 kg/m2 | 148 (41.0) | 95 (42.2) | 53 (39.0) | 0.54 |
| Total cholesterol, mmol/L | 4.99 ± 0.86 | 5.01 ± 0.87 | 4.95 ± 0.85 | 0.50 |
| Triglycerides, mmol/L | 1.69 ± 1.66 | 1.72 ± 1.72 | 1.64 ± 1.56 | 0.66 |
| HDL cholesterol, mmol/L | 1.55 ± 0.44 | 1.58 ± 0.47 | 1.51 ± 0.39 | 0.13 |
| LDL cholesterol, mmol/L | 2.82 ± 0.71 | 2.80 ± 0.71 | 2.86 ± 0.71 | 0.41 |
| Glucose, mmol/L | 5.89 ± 1.26 | 5.82 ± 1.22 | 6.02 ± 1.31 | 0.16 |
| HbA1c, % | 5.8 ± 0.6 | 5.7 ± 0.6 | 5.8 ± 0.6 | 0.09 |
| eGFR, ml/min/1.73 m2 | 66.9 ± 15.6 | 68.4 ± 15.1 | 64.4 ± 16.1 | 0.03 |
| Chronic kidney disease, n (%) | 102 (28.3) | 50 (22.2) | 52 (38.2) | 0.001 |
| Smoker, n (%) | 165 (47.8) | 102 (46.6) | 63 (50.0) | 0.54 |
| Comorbidities, n (%) | ||||
| Dyslipidemia | 300 (83.3) | 192 (85.7) | 108 (79.4) | 0.12 |
| Diabetes mellitus | 92 (25.5) | 54 (24.1) | 38 (27.7) | 0.44 |
| Coronary heart disease | 53 (14.7) | 27 (12.0) | 26 (19.1) | 0.06 |
| Cerebrovascular disease | 38 (10.6) | 15 (6.7) | 23 (16.9) | 0.002 |
| Sleep apnea syndrome | 34 (9.6) | 20 (9.1) | 14 (10.5) | 0.67 |
| Primary aldosteronism | 7 (1.9) | 4 (1.8) | 3 (2.2) | 0.78 |
| Medication use, n (%) | ||||
| Calcium channel blockers | 303 (83.0) | 196 (86.3) | 107 (77.5) | 0.03 |
| ARBs | 266 (72.9) | 165 (72.7) | 101 (73.2) | 0.92 |
| ACEIs | 13 (3.5) | 5 (2.2) | 8 (5.8) | 0.07 |
| β-blockers | 56 (15.3) | 28 (12.3) | 28 (20.3) | 0.04 |
| α-blockers | 7 (1.9) | 1 (0.4) | 6 (4.4) | 0.008 |
| Diuretics | 81 (22.2) | 42 (18.5) | 39 (28.3) | 0.03 |
| MRAs | 19 (5.2) | 13 (5.7) | 6 (4.4) | 0.57 |
| Lipid-lowering drugs | 184 (50.4) | 115 (50.7) | 69 (50.0) | 0.90 |
| Hypoglycemic drugs | 56 (15.3) | 29 (12.8) | 27 (19.6) | 0.08 |
| Bedtime administration of antihypertensive drugs | 145 (39.7) | 106 (40.5) | 53 (29.6) | 0.02 |
| Number of antihypertensive drugs | 2.0 ± 0.8 | 1.99 ± 0.74 | 2.16 ± 0.97 | 0.03 |
| Office systolic blood pressure, mm Hg | 130.1 ± 15.9 | 127.3 ± 14.4 | 134.6 ± 17.2 | <0.001 |
| Office diastolic blood pressure, mm Hg | 72.1 ± 13.6 | 71.0 ± 13.5 | 73.8 ± 13.6 | 0.06 |
| Office pulse rate, bpm | 72.1 ± 11.5 | 72.8 ± 11.4 | 71.0 ± 11.6 | 0.16 |
| Home blood pressure monitoring | ||||
| Nighttime systolic blood pressure, mm Hg | 117.4 ± 12.7 | 109.8 ± 6.6 | 130.9 ± 9.3 | N/A |
| Nighttime diastolic blood pressure, mm Hg | 69.5 ± 7.8 | 67.4 ± 6.9 | 73.3 ± 8.3 | <0.001 |
| Nighttime pulse rate, bpm | 61.3 ± 7.8 | 61.7 ± 7.6 | 60.7 ± 8.5 | 0.20 |
| Morning systolic blood pressure, mm Hg | 131.0 ± 13.3 | 125.6 ± 9.8 | 139.8 ± 13.6 | <0.001 |
| Morning diastolic blood pressure, mm Hg | 78.7 ± 9.6 | 77.9 ± 8.7 | 79.9 ± 10.8 | 0.04 |
| Morning pulse rate, bpm | 66.1 ± 9.2 | 67.0 ± 9.1 | 64.6 ± 9.1 | 0.02 |
| Evening systolic blood pressure, mm Hg | 125.3 ± 14.4 | 120.5 ± 11.8 | 133.1 ± 15.0 | <0.001 |
| Evening diastolic blood pressure, mm Hg | 72.9 ± 9.7 | 72.1 ± 9.0 | 74.2 ± 10.6 | 0.04 |
| Evening pulse rate, bpm | 70.1 ± 10.0 | 70.8 ± 9.3 | 68.9 ± 11.0 | 0.07 |
| Riser pattern, n (%) | 55 (15.1) | 11 (4.9) | 44 (31.9) | <0.001 |
| . | All . | Nonnocturnal hypertension . | Nocturnal hypertension . | . |
|---|---|---|---|---|
| Variables . | (n = 365) . | (n = 227) . | (n = 138) . | P value . |
| Age, years | 67.9 ± 10.3 | 66.2 ± 10.8 | 70.5 ± 9.0 | <0.001 |
| Age ≥ 65 years | 262 (71.8) | 149 (65.6) | 113 (81.9) | <0.001 |
| Men, n (%) | 224 (61.4) | 138 (60.8) | 86 (62.3) | 0.77 |
| Body mass index, kg/m2 | 24.6 ± 3.6 | 24.7 ± 3.4 | 24.6 ± 3.8 | 0.89 |
| Body mass index ≥ 25 kg/m2 | 148 (41.0) | 95 (42.2) | 53 (39.0) | 0.54 |
| Total cholesterol, mmol/L | 4.99 ± 0.86 | 5.01 ± 0.87 | 4.95 ± 0.85 | 0.50 |
| Triglycerides, mmol/L | 1.69 ± 1.66 | 1.72 ± 1.72 | 1.64 ± 1.56 | 0.66 |
| HDL cholesterol, mmol/L | 1.55 ± 0.44 | 1.58 ± 0.47 | 1.51 ± 0.39 | 0.13 |
| LDL cholesterol, mmol/L | 2.82 ± 0.71 | 2.80 ± 0.71 | 2.86 ± 0.71 | 0.41 |
| Glucose, mmol/L | 5.89 ± 1.26 | 5.82 ± 1.22 | 6.02 ± 1.31 | 0.16 |
| HbA1c, % | 5.8 ± 0.6 | 5.7 ± 0.6 | 5.8 ± 0.6 | 0.09 |
| eGFR, ml/min/1.73 m2 | 66.9 ± 15.6 | 68.4 ± 15.1 | 64.4 ± 16.1 | 0.03 |
| Chronic kidney disease, n (%) | 102 (28.3) | 50 (22.2) | 52 (38.2) | 0.001 |
| Smoker, n (%) | 165 (47.8) | 102 (46.6) | 63 (50.0) | 0.54 |
| Comorbidities, n (%) | ||||
| Dyslipidemia | 300 (83.3) | 192 (85.7) | 108 (79.4) | 0.12 |
| Diabetes mellitus | 92 (25.5) | 54 (24.1) | 38 (27.7) | 0.44 |
| Coronary heart disease | 53 (14.7) | 27 (12.0) | 26 (19.1) | 0.06 |
| Cerebrovascular disease | 38 (10.6) | 15 (6.7) | 23 (16.9) | 0.002 |
| Sleep apnea syndrome | 34 (9.6) | 20 (9.1) | 14 (10.5) | 0.67 |
| Primary aldosteronism | 7 (1.9) | 4 (1.8) | 3 (2.2) | 0.78 |
| Medication use, n (%) | ||||
| Calcium channel blockers | 303 (83.0) | 196 (86.3) | 107 (77.5) | 0.03 |
| ARBs | 266 (72.9) | 165 (72.7) | 101 (73.2) | 0.92 |
| ACEIs | 13 (3.5) | 5 (2.2) | 8 (5.8) | 0.07 |
| β-blockers | 56 (15.3) | 28 (12.3) | 28 (20.3) | 0.04 |
| α-blockers | 7 (1.9) | 1 (0.4) | 6 (4.4) | 0.008 |
| Diuretics | 81 (22.2) | 42 (18.5) | 39 (28.3) | 0.03 |
| MRAs | 19 (5.2) | 13 (5.7) | 6 (4.4) | 0.57 |
| Lipid-lowering drugs | 184 (50.4) | 115 (50.7) | 69 (50.0) | 0.90 |
| Hypoglycemic drugs | 56 (15.3) | 29 (12.8) | 27 (19.6) | 0.08 |
| Bedtime administration of antihypertensive drugs | 145 (39.7) | 106 (40.5) | 53 (29.6) | 0.02 |
| Number of antihypertensive drugs | 2.0 ± 0.8 | 1.99 ± 0.74 | 2.16 ± 0.97 | 0.03 |
| Office systolic blood pressure, mm Hg | 130.1 ± 15.9 | 127.3 ± 14.4 | 134.6 ± 17.2 | <0.001 |
| Office diastolic blood pressure, mm Hg | 72.1 ± 13.6 | 71.0 ± 13.5 | 73.8 ± 13.6 | 0.06 |
| Office pulse rate, bpm | 72.1 ± 11.5 | 72.8 ± 11.4 | 71.0 ± 11.6 | 0.16 |
| Home blood pressure monitoring | ||||
| Nighttime systolic blood pressure, mm Hg | 117.4 ± 12.7 | 109.8 ± 6.6 | 130.9 ± 9.3 | N/A |
| Nighttime diastolic blood pressure, mm Hg | 69.5 ± 7.8 | 67.4 ± 6.9 | 73.3 ± 8.3 | <0.001 |
| Nighttime pulse rate, bpm | 61.3 ± 7.8 | 61.7 ± 7.6 | 60.7 ± 8.5 | 0.20 |
| Morning systolic blood pressure, mm Hg | 131.0 ± 13.3 | 125.6 ± 9.8 | 139.8 ± 13.6 | <0.001 |
| Morning diastolic blood pressure, mm Hg | 78.7 ± 9.6 | 77.9 ± 8.7 | 79.9 ± 10.8 | 0.04 |
| Morning pulse rate, bpm | 66.1 ± 9.2 | 67.0 ± 9.1 | 64.6 ± 9.1 | 0.02 |
| Evening systolic blood pressure, mm Hg | 125.3 ± 14.4 | 120.5 ± 11.8 | 133.1 ± 15.0 | <0.001 |
| Evening diastolic blood pressure, mm Hg | 72.9 ± 9.7 | 72.1 ± 9.0 | 74.2 ± 10.6 | 0.04 |
| Evening pulse rate, bpm | 70.1 ± 10.0 | 70.8 ± 9.3 | 68.9 ± 11.0 | 0.07 |
| Riser pattern, n (%) | 55 (15.1) | 11 (4.9) | 44 (31.9) | <0.001 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; MRA, mineralocorticoid receptor antagonist.
| . | All . | Nonnocturnal hypertension . | Nocturnal hypertension . | . |
|---|---|---|---|---|
| Variables . | (n = 365) . | (n = 227) . | (n = 138) . | P value . |
| Age, years | 67.9 ± 10.3 | 66.2 ± 10.8 | 70.5 ± 9.0 | <0.001 |
| Age ≥ 65 years | 262 (71.8) | 149 (65.6) | 113 (81.9) | <0.001 |
| Men, n (%) | 224 (61.4) | 138 (60.8) | 86 (62.3) | 0.77 |
| Body mass index, kg/m2 | 24.6 ± 3.6 | 24.7 ± 3.4 | 24.6 ± 3.8 | 0.89 |
| Body mass index ≥ 25 kg/m2 | 148 (41.0) | 95 (42.2) | 53 (39.0) | 0.54 |
| Total cholesterol, mmol/L | 4.99 ± 0.86 | 5.01 ± 0.87 | 4.95 ± 0.85 | 0.50 |
| Triglycerides, mmol/L | 1.69 ± 1.66 | 1.72 ± 1.72 | 1.64 ± 1.56 | 0.66 |
| HDL cholesterol, mmol/L | 1.55 ± 0.44 | 1.58 ± 0.47 | 1.51 ± 0.39 | 0.13 |
| LDL cholesterol, mmol/L | 2.82 ± 0.71 | 2.80 ± 0.71 | 2.86 ± 0.71 | 0.41 |
| Glucose, mmol/L | 5.89 ± 1.26 | 5.82 ± 1.22 | 6.02 ± 1.31 | 0.16 |
| HbA1c, % | 5.8 ± 0.6 | 5.7 ± 0.6 | 5.8 ± 0.6 | 0.09 |
| eGFR, ml/min/1.73 m2 | 66.9 ± 15.6 | 68.4 ± 15.1 | 64.4 ± 16.1 | 0.03 |
| Chronic kidney disease, n (%) | 102 (28.3) | 50 (22.2) | 52 (38.2) | 0.001 |
| Smoker, n (%) | 165 (47.8) | 102 (46.6) | 63 (50.0) | 0.54 |
| Comorbidities, n (%) | ||||
| Dyslipidemia | 300 (83.3) | 192 (85.7) | 108 (79.4) | 0.12 |
| Diabetes mellitus | 92 (25.5) | 54 (24.1) | 38 (27.7) | 0.44 |
| Coronary heart disease | 53 (14.7) | 27 (12.0) | 26 (19.1) | 0.06 |
| Cerebrovascular disease | 38 (10.6) | 15 (6.7) | 23 (16.9) | 0.002 |
| Sleep apnea syndrome | 34 (9.6) | 20 (9.1) | 14 (10.5) | 0.67 |
| Primary aldosteronism | 7 (1.9) | 4 (1.8) | 3 (2.2) | 0.78 |
| Medication use, n (%) | ||||
| Calcium channel blockers | 303 (83.0) | 196 (86.3) | 107 (77.5) | 0.03 |
| ARBs | 266 (72.9) | 165 (72.7) | 101 (73.2) | 0.92 |
| ACEIs | 13 (3.5) | 5 (2.2) | 8 (5.8) | 0.07 |
| β-blockers | 56 (15.3) | 28 (12.3) | 28 (20.3) | 0.04 |
| α-blockers | 7 (1.9) | 1 (0.4) | 6 (4.4) | 0.008 |
| Diuretics | 81 (22.2) | 42 (18.5) | 39 (28.3) | 0.03 |
| MRAs | 19 (5.2) | 13 (5.7) | 6 (4.4) | 0.57 |
| Lipid-lowering drugs | 184 (50.4) | 115 (50.7) | 69 (50.0) | 0.90 |
| Hypoglycemic drugs | 56 (15.3) | 29 (12.8) | 27 (19.6) | 0.08 |
| Bedtime administration of antihypertensive drugs | 145 (39.7) | 106 (40.5) | 53 (29.6) | 0.02 |
| Number of antihypertensive drugs | 2.0 ± 0.8 | 1.99 ± 0.74 | 2.16 ± 0.97 | 0.03 |
| Office systolic blood pressure, mm Hg | 130.1 ± 15.9 | 127.3 ± 14.4 | 134.6 ± 17.2 | <0.001 |
| Office diastolic blood pressure, mm Hg | 72.1 ± 13.6 | 71.0 ± 13.5 | 73.8 ± 13.6 | 0.06 |
| Office pulse rate, bpm | 72.1 ± 11.5 | 72.8 ± 11.4 | 71.0 ± 11.6 | 0.16 |
| Home blood pressure monitoring | ||||
| Nighttime systolic blood pressure, mm Hg | 117.4 ± 12.7 | 109.8 ± 6.6 | 130.9 ± 9.3 | N/A |
| Nighttime diastolic blood pressure, mm Hg | 69.5 ± 7.8 | 67.4 ± 6.9 | 73.3 ± 8.3 | <0.001 |
| Nighttime pulse rate, bpm | 61.3 ± 7.8 | 61.7 ± 7.6 | 60.7 ± 8.5 | 0.20 |
| Morning systolic blood pressure, mm Hg | 131.0 ± 13.3 | 125.6 ± 9.8 | 139.8 ± 13.6 | <0.001 |
| Morning diastolic blood pressure, mm Hg | 78.7 ± 9.6 | 77.9 ± 8.7 | 79.9 ± 10.8 | 0.04 |
| Morning pulse rate, bpm | 66.1 ± 9.2 | 67.0 ± 9.1 | 64.6 ± 9.1 | 0.02 |
| Evening systolic blood pressure, mm Hg | 125.3 ± 14.4 | 120.5 ± 11.8 | 133.1 ± 15.0 | <0.001 |
| Evening diastolic blood pressure, mm Hg | 72.9 ± 9.7 | 72.1 ± 9.0 | 74.2 ± 10.6 | 0.04 |
| Evening pulse rate, bpm | 70.1 ± 10.0 | 70.8 ± 9.3 | 68.9 ± 11.0 | 0.07 |
| Riser pattern, n (%) | 55 (15.1) | 11 (4.9) | 44 (31.9) | <0.001 |
| . | All . | Nonnocturnal hypertension . | Nocturnal hypertension . | . |
|---|---|---|---|---|
| Variables . | (n = 365) . | (n = 227) . | (n = 138) . | P value . |
| Age, years | 67.9 ± 10.3 | 66.2 ± 10.8 | 70.5 ± 9.0 | <0.001 |
| Age ≥ 65 years | 262 (71.8) | 149 (65.6) | 113 (81.9) | <0.001 |
| Men, n (%) | 224 (61.4) | 138 (60.8) | 86 (62.3) | 0.77 |
| Body mass index, kg/m2 | 24.6 ± 3.6 | 24.7 ± 3.4 | 24.6 ± 3.8 | 0.89 |
| Body mass index ≥ 25 kg/m2 | 148 (41.0) | 95 (42.2) | 53 (39.0) | 0.54 |
| Total cholesterol, mmol/L | 4.99 ± 0.86 | 5.01 ± 0.87 | 4.95 ± 0.85 | 0.50 |
| Triglycerides, mmol/L | 1.69 ± 1.66 | 1.72 ± 1.72 | 1.64 ± 1.56 | 0.66 |
| HDL cholesterol, mmol/L | 1.55 ± 0.44 | 1.58 ± 0.47 | 1.51 ± 0.39 | 0.13 |
| LDL cholesterol, mmol/L | 2.82 ± 0.71 | 2.80 ± 0.71 | 2.86 ± 0.71 | 0.41 |
| Glucose, mmol/L | 5.89 ± 1.26 | 5.82 ± 1.22 | 6.02 ± 1.31 | 0.16 |
| HbA1c, % | 5.8 ± 0.6 | 5.7 ± 0.6 | 5.8 ± 0.6 | 0.09 |
| eGFR, ml/min/1.73 m2 | 66.9 ± 15.6 | 68.4 ± 15.1 | 64.4 ± 16.1 | 0.03 |
| Chronic kidney disease, n (%) | 102 (28.3) | 50 (22.2) | 52 (38.2) | 0.001 |
| Smoker, n (%) | 165 (47.8) | 102 (46.6) | 63 (50.0) | 0.54 |
| Comorbidities, n (%) | ||||
| Dyslipidemia | 300 (83.3) | 192 (85.7) | 108 (79.4) | 0.12 |
| Diabetes mellitus | 92 (25.5) | 54 (24.1) | 38 (27.7) | 0.44 |
| Coronary heart disease | 53 (14.7) | 27 (12.0) | 26 (19.1) | 0.06 |
| Cerebrovascular disease | 38 (10.6) | 15 (6.7) | 23 (16.9) | 0.002 |
| Sleep apnea syndrome | 34 (9.6) | 20 (9.1) | 14 (10.5) | 0.67 |
| Primary aldosteronism | 7 (1.9) | 4 (1.8) | 3 (2.2) | 0.78 |
| Medication use, n (%) | ||||
| Calcium channel blockers | 303 (83.0) | 196 (86.3) | 107 (77.5) | 0.03 |
| ARBs | 266 (72.9) | 165 (72.7) | 101 (73.2) | 0.92 |
| ACEIs | 13 (3.5) | 5 (2.2) | 8 (5.8) | 0.07 |
| β-blockers | 56 (15.3) | 28 (12.3) | 28 (20.3) | 0.04 |
| α-blockers | 7 (1.9) | 1 (0.4) | 6 (4.4) | 0.008 |
| Diuretics | 81 (22.2) | 42 (18.5) | 39 (28.3) | 0.03 |
| MRAs | 19 (5.2) | 13 (5.7) | 6 (4.4) | 0.57 |
| Lipid-lowering drugs | 184 (50.4) | 115 (50.7) | 69 (50.0) | 0.90 |
| Hypoglycemic drugs | 56 (15.3) | 29 (12.8) | 27 (19.6) | 0.08 |
| Bedtime administration of antihypertensive drugs | 145 (39.7) | 106 (40.5) | 53 (29.6) | 0.02 |
| Number of antihypertensive drugs | 2.0 ± 0.8 | 1.99 ± 0.74 | 2.16 ± 0.97 | 0.03 |
| Office systolic blood pressure, mm Hg | 130.1 ± 15.9 | 127.3 ± 14.4 | 134.6 ± 17.2 | <0.001 |
| Office diastolic blood pressure, mm Hg | 72.1 ± 13.6 | 71.0 ± 13.5 | 73.8 ± 13.6 | 0.06 |
| Office pulse rate, bpm | 72.1 ± 11.5 | 72.8 ± 11.4 | 71.0 ± 11.6 | 0.16 |
| Home blood pressure monitoring | ||||
| Nighttime systolic blood pressure, mm Hg | 117.4 ± 12.7 | 109.8 ± 6.6 | 130.9 ± 9.3 | N/A |
| Nighttime diastolic blood pressure, mm Hg | 69.5 ± 7.8 | 67.4 ± 6.9 | 73.3 ± 8.3 | <0.001 |
| Nighttime pulse rate, bpm | 61.3 ± 7.8 | 61.7 ± 7.6 | 60.7 ± 8.5 | 0.20 |
| Morning systolic blood pressure, mm Hg | 131.0 ± 13.3 | 125.6 ± 9.8 | 139.8 ± 13.6 | <0.001 |
| Morning diastolic blood pressure, mm Hg | 78.7 ± 9.6 | 77.9 ± 8.7 | 79.9 ± 10.8 | 0.04 |
| Morning pulse rate, bpm | 66.1 ± 9.2 | 67.0 ± 9.1 | 64.6 ± 9.1 | 0.02 |
| Evening systolic blood pressure, mm Hg | 125.3 ± 14.4 | 120.5 ± 11.8 | 133.1 ± 15.0 | <0.001 |
| Evening diastolic blood pressure, mm Hg | 72.9 ± 9.7 | 72.1 ± 9.0 | 74.2 ± 10.6 | 0.04 |
| Evening pulse rate, bpm | 70.1 ± 10.0 | 70.8 ± 9.3 | 68.9 ± 11.0 | 0.07 |
| Riser pattern, n (%) | 55 (15.1) | 11 (4.9) | 44 (31.9) | <0.001 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; MRA, mineralocorticoid receptor antagonist.
Associations of nighttime systolic blood pressure with office systolic blood pressure, morning systolic blood pressure, and evening systolic blood pressure
Univariate regression analysis showed that nighttime systolic BP correlated significantly with morning systolic BP (r = 0.61, P < 0.001), evening systolic BP (r = 0.57, P < 0.001), and office systolic BP (r = 0.32, P < 0.001).
Of the 365 subjects, 138 (37.8%) had nocturnal hypertension. The clinical characteristics of subjects based on nocturnal hypertension are summarized in Table 1. ROC curves of morning systolic BP, evening systolic BP, and office systolic BP for predicting subjects with nocturnal hypertension are shown in Figure 1. The AUC value of the ROC curve for morning systolic BP to predict nocturnal hypertension was significantly higher than that for evening systolic BP (0.81 vs. 0.75, P = 0.04) and that for office systolic BP (0.81 vs. 0.63, P < 0.001). The ROC curve analysis showed that the optimal cutoff value of morning systolic BP to diagnose subjects with nocturnal hypertension was 134.8 mm Hg with sensitivity of 0.65 and specificity of 0.84.
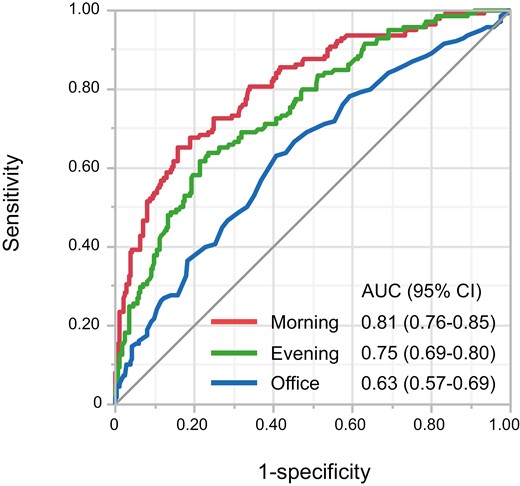
Receiver operating characteristic curves of morning systolic BP, evening BP, and office BP to discriminate subjects with nocturnal hypertension. Abbreviations: AUC, area under the curve; CI, confidence interval.
We categorized the subjects into 3 groups according to morning systolic BP: subjects with morning systolic BP of < 125 mm Hg (n = 127), subjects with morning systolic BP of 125–<135 mm Hg (n = 114), and subjects with morning systolic BP of ≥ 135 mm Hg (n = 124). The prevalences of nocturnal hypertension were 13.4% in subjects with morning systolic BP of < 125 mm Hg, 29.0% in subjects with morning systolic BP of 125–<135 mm Hg, and 71.0% in subjects with morning systolic BP of ≥ 135 mm Hg (P < 0.001) (Figure 2).
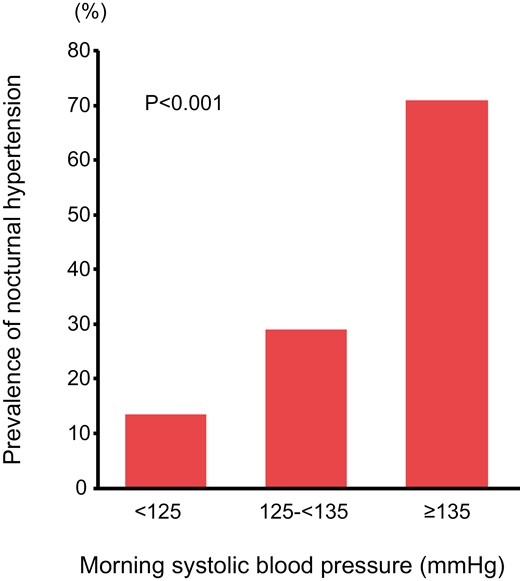
Bar graphs show the prevalences of nocturnal hypertension in subjects with morning systolic BP of < 125 mm Hg, subjects with morning systolic BP of 125–<135 mm Hg, and subjects with morning systolic BP of > 135 mm Hg.
Relationships between nocturnal hypertension and clinical variables
Univariate logistic regression analysis showed that office systolic BP of 130–<140 mm Hg (odds ratio [OR], 2.12; 95% confidence interval [CI], 1.26–3.57; P = 0.005), office systolic BP of ≥ 140 mm Hg (OR, 2.88; 95% CI, 1.69–4.91; P < 0.001), morning systolic BP of 125–<135 mm Hg (OR, 2.64; 95% CI, 1.37–5.06; P = 0.004), morning systolic BP of ≥ 135 mm Hg (OR, 15.8; 95% CI, 8.33–30.0; P < 0.001), evening systolic BP of 125–<135 mm Hg (OR, 2.80; 95% CI, 1.64–4.79; P < 0.001), evening systolic BP of ≥ 135 mm Hg (OR, 7.75; 95% CI, 4.32–13.9; P < 0.001), age ≥ 65 years (OR, 2.37; 95% CI, 1.42–3.95; P = 0.001), and a history of cerebrovascular disease (OR, 2.84; 95% CI, 1.42–5.65; P = 0.003) were significantly related to a higher risk of nocturnal hypertension and that eGFR (OR, 0.98; 95% CI, 0.970–0.998; P = 0.02) was significantly related to a lower risk of nocturnal hypertension (Table 2). A history of CHD (P = 0.07), bedtime dosing of antihypertensive drugs (P = 0.05), and number of antihypertensive drugs (P = 0.08) had borderline significance. Multivariate logistic regression analysis revealed that morning systolic BP of 125–<135 mm Hg (OR, 2.26; 95% CI, 1.13–4.58; P = 0.02), morning systolic BP of ≥ 135 mm Hg (OR, 16.4; 95% CI, 8.20–32.7; P < 0.001), and a history of cerebrovascular disease (OR, 3.99; 95% CI, 1.75–9.13; P = 0.001) were significantly associated with a higher risk of nocturnal hypertension and that bedtime dosing of antihypertensive drugs was significantly associated with a lower risk of nocturnal hypertension (OR, 0.56; 95% CI, 0.32–0.97; P = 0.04) (Table 3).
Univariate logistic regression analysis and multivariate logistic regression analysis of the relationships between nocturnal hypertension and variables
| . | Univariate logistic regression . | Multivariate logistic regression . | ||
|---|---|---|---|---|
| Covariates . | Odds ratio (95% CI) . | P value . | Odds ratio (95% CI) . | P value . |
| Age ≥ 65 years | 2.37 (1.42–3.95) | 0.001 | 1.81 (0.95–3.43) | 0.07 |
| Men | 1.07 (0.69–1.65) | 0.77 | 0.79 (0.45–1.38) | 0.51 |
| Body mass index ≥ 25 | 0.87 (0.57–1.35) | 0.54 | ||
| Total cholesterol, mmol/L | 0.91 (0.71–1.18) | 0.50 | ||
| Triglycerides, mmol/L | 0.97 (0.85–1.11) | 0.66 | ||
| HDL cholesterol, mmol/L | 0.67 (0.40–1.12) | 0.12 | ||
| LDL cholesterol, mmol/L | 1.14 (0.84–1.54) | 0.41 | ||
| Glucose, mmol/L | 1.13 (0.95–1.35) | 0.16 | ||
| HbA1c, % | 1.38 (0.95–1.99) | 0.99 | ||
| eGFR, ml/min/1.73 m2 | 0.98 (0.970–0.998) | 0.02 | ||
| Chronic kidney disease | 2.17 (1.36–3.46) | 0.001 | 1.60 (0.88–2.90) | 0.12 |
| Smokers | 1.15 (0.74–1.78) | 0.54 | ||
| Dyslipidemia | 0.64 (0.37–1.12) | 0.12 | ||
| Diabetes mellitus | 1.21 (0.75–1.96) | 0.44 | ||
| Coronary heart disease | 1.73 (0.96–3.12) | 0.07 | 1.45 (0.68–3.09) | 0.34 |
| Cerebrovascular disease | 2.84 (1.42–5.65) | 0.003 | 3.99 (1.75–9.13) | 0.001 |
| Sleep apnea syndrome | 0.72 (0.21–2.43) | 0.60 | ||
| Primary aldosteronism | 1.24 (0.27–5.63) | 0.78 | ||
| Bedtime dosing of antihypertensive drugs | 0.65 (0.42–1.00) | 0.05 | 0.56 (0.32–0.97) | 0.04 |
| Number of antihypertensive drugs | 1.25 (0.97–1.61) | 0.08 | 1.16 (0.83–1.62) | 0.39 |
| Office systolic blood pressure < 130 mm Hg | 1 (reference) | |||
| Office systolic blood pressure 130–<140 mm Hg | 2.12 (1.26–3.57) | 0.005 | ||
| Office systolic blood pressure ≥ 140 mm Hg | 2.88 (1.69–4.91) | <0.001 | ||
| Morning systolic blood pressure < 125 mm Hg | 1 (reference) | 1 (reference) | ||
| Morning systolic blood pressure 125–<135 mm Hg | 2.64 (1.37–5.06) | 0.004 | 2.26 (1.13–4.58) | 0.02 |
| Morning systolic blood pressure ≥ 135 mm Hg | 15.8 (8.33–30.0) | <0.001 | 16.4 (8.20–32.7) | <0.001 |
| Evening systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Evening systolic blood pressure 125–<135 mm Hg | 2.80 (1.64–4.79) | <0.001 | ||
| Evening systolic blood pressure ≥ 135 mm Hg | 7.75 (4.32–13.9) | <0.001 | ||
| . | Univariate logistic regression . | Multivariate logistic regression . | ||
|---|---|---|---|---|
| Covariates . | Odds ratio (95% CI) . | P value . | Odds ratio (95% CI) . | P value . |
| Age ≥ 65 years | 2.37 (1.42–3.95) | 0.001 | 1.81 (0.95–3.43) | 0.07 |
| Men | 1.07 (0.69–1.65) | 0.77 | 0.79 (0.45–1.38) | 0.51 |
| Body mass index ≥ 25 | 0.87 (0.57–1.35) | 0.54 | ||
| Total cholesterol, mmol/L | 0.91 (0.71–1.18) | 0.50 | ||
| Triglycerides, mmol/L | 0.97 (0.85–1.11) | 0.66 | ||
| HDL cholesterol, mmol/L | 0.67 (0.40–1.12) | 0.12 | ||
| LDL cholesterol, mmol/L | 1.14 (0.84–1.54) | 0.41 | ||
| Glucose, mmol/L | 1.13 (0.95–1.35) | 0.16 | ||
| HbA1c, % | 1.38 (0.95–1.99) | 0.99 | ||
| eGFR, ml/min/1.73 m2 | 0.98 (0.970–0.998) | 0.02 | ||
| Chronic kidney disease | 2.17 (1.36–3.46) | 0.001 | 1.60 (0.88–2.90) | 0.12 |
| Smokers | 1.15 (0.74–1.78) | 0.54 | ||
| Dyslipidemia | 0.64 (0.37–1.12) | 0.12 | ||
| Diabetes mellitus | 1.21 (0.75–1.96) | 0.44 | ||
| Coronary heart disease | 1.73 (0.96–3.12) | 0.07 | 1.45 (0.68–3.09) | 0.34 |
| Cerebrovascular disease | 2.84 (1.42–5.65) | 0.003 | 3.99 (1.75–9.13) | 0.001 |
| Sleep apnea syndrome | 0.72 (0.21–2.43) | 0.60 | ||
| Primary aldosteronism | 1.24 (0.27–5.63) | 0.78 | ||
| Bedtime dosing of antihypertensive drugs | 0.65 (0.42–1.00) | 0.05 | 0.56 (0.32–0.97) | 0.04 |
| Number of antihypertensive drugs | 1.25 (0.97–1.61) | 0.08 | 1.16 (0.83–1.62) | 0.39 |
| Office systolic blood pressure < 130 mm Hg | 1 (reference) | |||
| Office systolic blood pressure 130–<140 mm Hg | 2.12 (1.26–3.57) | 0.005 | ||
| Office systolic blood pressure ≥ 140 mm Hg | 2.88 (1.69–4.91) | <0.001 | ||
| Morning systolic blood pressure < 125 mm Hg | 1 (reference) | 1 (reference) | ||
| Morning systolic blood pressure 125–<135 mm Hg | 2.64 (1.37–5.06) | 0.004 | 2.26 (1.13–4.58) | 0.02 |
| Morning systolic blood pressure ≥ 135 mm Hg | 15.8 (8.33–30.0) | <0.001 | 16.4 (8.20–32.7) | <0.001 |
| Evening systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Evening systolic blood pressure 125–<135 mm Hg | 2.80 (1.64–4.79) | <0.001 | ||
| Evening systolic blood pressure ≥ 135 mm Hg | 7.75 (4.32–13.9) | <0.001 | ||
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate.
Univariate logistic regression analysis and multivariate logistic regression analysis of the relationships between nocturnal hypertension and variables
| . | Univariate logistic regression . | Multivariate logistic regression . | ||
|---|---|---|---|---|
| Covariates . | Odds ratio (95% CI) . | P value . | Odds ratio (95% CI) . | P value . |
| Age ≥ 65 years | 2.37 (1.42–3.95) | 0.001 | 1.81 (0.95–3.43) | 0.07 |
| Men | 1.07 (0.69–1.65) | 0.77 | 0.79 (0.45–1.38) | 0.51 |
| Body mass index ≥ 25 | 0.87 (0.57–1.35) | 0.54 | ||
| Total cholesterol, mmol/L | 0.91 (0.71–1.18) | 0.50 | ||
| Triglycerides, mmol/L | 0.97 (0.85–1.11) | 0.66 | ||
| HDL cholesterol, mmol/L | 0.67 (0.40–1.12) | 0.12 | ||
| LDL cholesterol, mmol/L | 1.14 (0.84–1.54) | 0.41 | ||
| Glucose, mmol/L | 1.13 (0.95–1.35) | 0.16 | ||
| HbA1c, % | 1.38 (0.95–1.99) | 0.99 | ||
| eGFR, ml/min/1.73 m2 | 0.98 (0.970–0.998) | 0.02 | ||
| Chronic kidney disease | 2.17 (1.36–3.46) | 0.001 | 1.60 (0.88–2.90) | 0.12 |
| Smokers | 1.15 (0.74–1.78) | 0.54 | ||
| Dyslipidemia | 0.64 (0.37–1.12) | 0.12 | ||
| Diabetes mellitus | 1.21 (0.75–1.96) | 0.44 | ||
| Coronary heart disease | 1.73 (0.96–3.12) | 0.07 | 1.45 (0.68–3.09) | 0.34 |
| Cerebrovascular disease | 2.84 (1.42–5.65) | 0.003 | 3.99 (1.75–9.13) | 0.001 |
| Sleep apnea syndrome | 0.72 (0.21–2.43) | 0.60 | ||
| Primary aldosteronism | 1.24 (0.27–5.63) | 0.78 | ||
| Bedtime dosing of antihypertensive drugs | 0.65 (0.42–1.00) | 0.05 | 0.56 (0.32–0.97) | 0.04 |
| Number of antihypertensive drugs | 1.25 (0.97–1.61) | 0.08 | 1.16 (0.83–1.62) | 0.39 |
| Office systolic blood pressure < 130 mm Hg | 1 (reference) | |||
| Office systolic blood pressure 130–<140 mm Hg | 2.12 (1.26–3.57) | 0.005 | ||
| Office systolic blood pressure ≥ 140 mm Hg | 2.88 (1.69–4.91) | <0.001 | ||
| Morning systolic blood pressure < 125 mm Hg | 1 (reference) | 1 (reference) | ||
| Morning systolic blood pressure 125–<135 mm Hg | 2.64 (1.37–5.06) | 0.004 | 2.26 (1.13–4.58) | 0.02 |
| Morning systolic blood pressure ≥ 135 mm Hg | 15.8 (8.33–30.0) | <0.001 | 16.4 (8.20–32.7) | <0.001 |
| Evening systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Evening systolic blood pressure 125–<135 mm Hg | 2.80 (1.64–4.79) | <0.001 | ||
| Evening systolic blood pressure ≥ 135 mm Hg | 7.75 (4.32–13.9) | <0.001 | ||
| . | Univariate logistic regression . | Multivariate logistic regression . | ||
|---|---|---|---|---|
| Covariates . | Odds ratio (95% CI) . | P value . | Odds ratio (95% CI) . | P value . |
| Age ≥ 65 years | 2.37 (1.42–3.95) | 0.001 | 1.81 (0.95–3.43) | 0.07 |
| Men | 1.07 (0.69–1.65) | 0.77 | 0.79 (0.45–1.38) | 0.51 |
| Body mass index ≥ 25 | 0.87 (0.57–1.35) | 0.54 | ||
| Total cholesterol, mmol/L | 0.91 (0.71–1.18) | 0.50 | ||
| Triglycerides, mmol/L | 0.97 (0.85–1.11) | 0.66 | ||
| HDL cholesterol, mmol/L | 0.67 (0.40–1.12) | 0.12 | ||
| LDL cholesterol, mmol/L | 1.14 (0.84–1.54) | 0.41 | ||
| Glucose, mmol/L | 1.13 (0.95–1.35) | 0.16 | ||
| HbA1c, % | 1.38 (0.95–1.99) | 0.99 | ||
| eGFR, ml/min/1.73 m2 | 0.98 (0.970–0.998) | 0.02 | ||
| Chronic kidney disease | 2.17 (1.36–3.46) | 0.001 | 1.60 (0.88–2.90) | 0.12 |
| Smokers | 1.15 (0.74–1.78) | 0.54 | ||
| Dyslipidemia | 0.64 (0.37–1.12) | 0.12 | ||
| Diabetes mellitus | 1.21 (0.75–1.96) | 0.44 | ||
| Coronary heart disease | 1.73 (0.96–3.12) | 0.07 | 1.45 (0.68–3.09) | 0.34 |
| Cerebrovascular disease | 2.84 (1.42–5.65) | 0.003 | 3.99 (1.75–9.13) | 0.001 |
| Sleep apnea syndrome | 0.72 (0.21–2.43) | 0.60 | ||
| Primary aldosteronism | 1.24 (0.27–5.63) | 0.78 | ||
| Bedtime dosing of antihypertensive drugs | 0.65 (0.42–1.00) | 0.05 | 0.56 (0.32–0.97) | 0.04 |
| Number of antihypertensive drugs | 1.25 (0.97–1.61) | 0.08 | 1.16 (0.83–1.62) | 0.39 |
| Office systolic blood pressure < 130 mm Hg | 1 (reference) | |||
| Office systolic blood pressure 130–<140 mm Hg | 2.12 (1.26–3.57) | 0.005 | ||
| Office systolic blood pressure ≥ 140 mm Hg | 2.88 (1.69–4.91) | <0.001 | ||
| Morning systolic blood pressure < 125 mm Hg | 1 (reference) | 1 (reference) | ||
| Morning systolic blood pressure 125–<135 mm Hg | 2.64 (1.37–5.06) | 0.004 | 2.26 (1.13–4.58) | 0.02 |
| Morning systolic blood pressure ≥ 135 mm Hg | 15.8 (8.33–30.0) | <0.001 | 16.4 (8.20–32.7) | <0.001 |
| Evening systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Evening systolic blood pressure 125–<135 mm Hg | 2.80 (1.64–4.79) | <0.001 | ||
| Evening systolic blood pressure ≥ 135 mm Hg | 7.75 (4.32–13.9) | <0.001 | ||
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate.
| . | Non-riser pattern . | Riser pattern . | . |
|---|---|---|---|
| Variables . | (n = 310) . | (n = 55) . | P value . |
| Age, years | 67.2 ± 10.6 | 71.9 ± 7.7 | 0.002 |
| Age ≥ 65 years | 215 (69.4) | 47 (85.5) | 0.01 |
| Men, n (%) | 192 (61.9) | 32 (58.2) | 0.60 |
| Body mass index, kg/m2 | 24.8 (3.5) | 23.9 (3.7) | 0.10 |
| Body mass index ≥ 25 kg/m2 | 131 (42.7) | 17 (31.5) | 0.12 |
| Total cholesterol, mmol/L | 5.01 ± 0.87 | 4.83 ± 0.80 | 0.16 |
| Triglycerides, mmol/L | 1.71 ± 1.73 | 1.56 ± 1.18 | 0.57 |
| HDL cholesterol, mmol/L | 1.57 ± 0.45 | 1.45 ± 0.40 | 0.07 |
| LDL cholesterol, mmol/L | 2.84 ± 0.72 | 2.69 ± 0.61 | 0.16 |
| Glucose, mmol/L | 5.92 ± 1.26 | 5.72 ± 1.25 | 0.32 |
| HbA1c, % | 5.7 ± 0.6 | 5.8 ± 0.7 | 0.61 |
| eGFR, ml/min/1.73 m2 | 67.6 ± 15.5 | 63.0 ± 15.8 | 0.06 |
| Chronic kidney disease, n (%) | 82 (26.6) | 20 (37.7) | 0.10 |
| Smoker, n (%) | 139 (47.4) | 26 (50.0) | 0.73 |
| Comorbidities, n (%) | |||
| Dyslipidemia | 253 (82.7) | 47 (87.0) | 0.43 |
| Diabetes mellitus | 81 (26.4) | 11 (20.4) | 0.35 |
| Coronary heart disease | 46 (15.0) | 7 (13.0) | 0.70 |
| Cerebrovascular disease | 28 (9.2) | 10 (18.5) | 0.04 |
| Sleep apnea syndrome | 30 (10.0) | 4 (7.6) | 0.58 |
| Primary aldosteronism | 6 (2.0) | 1 (1.9) | 0.97 |
| Medication use, n (%) | |||
| Calcium channel blockers | 259 (83.6) | 44 (80.0) | 0.52 |
| ARBs | 227 (73.2) | 39 (70.9) | 0.72 |
| ACEIs | 11 (3.6) | 2 (3.6) | 0.97 |
| β-blockers | 48 (15.5) | 8 (14.6) | 0.86 |
| α-blockers | 5 (1.6) | 2 (3.6) | 0.31 |
| Diuretics | 65 (21.0) | 16 (29.1) | 0.18 |
| MRAs | 17 (5.5) | 2 (3.6) | 0.57 |
| Lipid-lowering drugs | 156 (50.3) | 28 (50.9) | 0.94 |
| Hypoglycemic drugs | 48 (15.5) | 8 (14.6) | 0.86 |
| Bedtime administration of antihypertensive drugs | 124 (40.0) | 21 (38.2) | 0.80 |
| Number of antihypertensive drugs | 2.0 ± 0.8 | 2.1 ± 1.0 | 0.90 |
| Office systolic blood pressure, mm Hg | 130.1 ± 16.2 | 130.0 ± 13.8 | 0.93 |
| Office diastolic blood pressure, mm Hg | 72.1 ± 14.0 | 71.7 ± 11.1 | 0.83 |
| Office pulse rate, bpm | 72.3 ± 11.4 | 70.8 ± 12.1 | 0.36 |
| Home blood pressure monitoring | |||
| Nighttime systolic blood pressure, mm Hg | 115.3 ± 11.6 | 129.6 ± 11.6 | <0.001 |
| Nighttime diastolic blood pressure, mm Hg | 68.9 ± 7.8 | 72.5 ± 7.3 | 0.002 |
| Nighttime pulse rate, bpm | 61.3 ± 7.7 | 61.4 ± 8.7 | 0.96 |
| Morning systolic blood pressure, mm Hg | 131.3 ± 13.4 | 128.9 ± 12.4 | 0.21 |
| Morning diastolic blood pressure, mm Hg | 79.2 ± 9.4 | 75.7 ± 10.1 | 0.01 |
| Morning pulse rate, bpm | 66.4 ± 9.3 | 64.4 ± 8.4 | 0.13 |
| Evening systolic blood pressure, mm Hg | 126.0 ± 14.6 | 121.3 ± 12.7 | 0.03 |
| Evening diastolic blood pressure, mm Hg | 73.6 ± 9.6 | 69.1 ± 9.4 | 0.001 |
| Evening pulse rate, bpm | 70.3 ± 9.9 | 69.1 ± 10.8 | 0.43 |
| . | Non-riser pattern . | Riser pattern . | . |
|---|---|---|---|
| Variables . | (n = 310) . | (n = 55) . | P value . |
| Age, years | 67.2 ± 10.6 | 71.9 ± 7.7 | 0.002 |
| Age ≥ 65 years | 215 (69.4) | 47 (85.5) | 0.01 |
| Men, n (%) | 192 (61.9) | 32 (58.2) | 0.60 |
| Body mass index, kg/m2 | 24.8 (3.5) | 23.9 (3.7) | 0.10 |
| Body mass index ≥ 25 kg/m2 | 131 (42.7) | 17 (31.5) | 0.12 |
| Total cholesterol, mmol/L | 5.01 ± 0.87 | 4.83 ± 0.80 | 0.16 |
| Triglycerides, mmol/L | 1.71 ± 1.73 | 1.56 ± 1.18 | 0.57 |
| HDL cholesterol, mmol/L | 1.57 ± 0.45 | 1.45 ± 0.40 | 0.07 |
| LDL cholesterol, mmol/L | 2.84 ± 0.72 | 2.69 ± 0.61 | 0.16 |
| Glucose, mmol/L | 5.92 ± 1.26 | 5.72 ± 1.25 | 0.32 |
| HbA1c, % | 5.7 ± 0.6 | 5.8 ± 0.7 | 0.61 |
| eGFR, ml/min/1.73 m2 | 67.6 ± 15.5 | 63.0 ± 15.8 | 0.06 |
| Chronic kidney disease, n (%) | 82 (26.6) | 20 (37.7) | 0.10 |
| Smoker, n (%) | 139 (47.4) | 26 (50.0) | 0.73 |
| Comorbidities, n (%) | |||
| Dyslipidemia | 253 (82.7) | 47 (87.0) | 0.43 |
| Diabetes mellitus | 81 (26.4) | 11 (20.4) | 0.35 |
| Coronary heart disease | 46 (15.0) | 7 (13.0) | 0.70 |
| Cerebrovascular disease | 28 (9.2) | 10 (18.5) | 0.04 |
| Sleep apnea syndrome | 30 (10.0) | 4 (7.6) | 0.58 |
| Primary aldosteronism | 6 (2.0) | 1 (1.9) | 0.97 |
| Medication use, n (%) | |||
| Calcium channel blockers | 259 (83.6) | 44 (80.0) | 0.52 |
| ARBs | 227 (73.2) | 39 (70.9) | 0.72 |
| ACEIs | 11 (3.6) | 2 (3.6) | 0.97 |
| β-blockers | 48 (15.5) | 8 (14.6) | 0.86 |
| α-blockers | 5 (1.6) | 2 (3.6) | 0.31 |
| Diuretics | 65 (21.0) | 16 (29.1) | 0.18 |
| MRAs | 17 (5.5) | 2 (3.6) | 0.57 |
| Lipid-lowering drugs | 156 (50.3) | 28 (50.9) | 0.94 |
| Hypoglycemic drugs | 48 (15.5) | 8 (14.6) | 0.86 |
| Bedtime administration of antihypertensive drugs | 124 (40.0) | 21 (38.2) | 0.80 |
| Number of antihypertensive drugs | 2.0 ± 0.8 | 2.1 ± 1.0 | 0.90 |
| Office systolic blood pressure, mm Hg | 130.1 ± 16.2 | 130.0 ± 13.8 | 0.93 |
| Office diastolic blood pressure, mm Hg | 72.1 ± 14.0 | 71.7 ± 11.1 | 0.83 |
| Office pulse rate, bpm | 72.3 ± 11.4 | 70.8 ± 12.1 | 0.36 |
| Home blood pressure monitoring | |||
| Nighttime systolic blood pressure, mm Hg | 115.3 ± 11.6 | 129.6 ± 11.6 | <0.001 |
| Nighttime diastolic blood pressure, mm Hg | 68.9 ± 7.8 | 72.5 ± 7.3 | 0.002 |
| Nighttime pulse rate, bpm | 61.3 ± 7.7 | 61.4 ± 8.7 | 0.96 |
| Morning systolic blood pressure, mm Hg | 131.3 ± 13.4 | 128.9 ± 12.4 | 0.21 |
| Morning diastolic blood pressure, mm Hg | 79.2 ± 9.4 | 75.7 ± 10.1 | 0.01 |
| Morning pulse rate, bpm | 66.4 ± 9.3 | 64.4 ± 8.4 | 0.13 |
| Evening systolic blood pressure, mm Hg | 126.0 ± 14.6 | 121.3 ± 12.7 | 0.03 |
| Evening diastolic blood pressure, mm Hg | 73.6 ± 9.6 | 69.1 ± 9.4 | 0.001 |
| Evening pulse rate, bpm | 70.3 ± 9.9 | 69.1 ± 10.8 | 0.43 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; MRA, mineralocorticoid receptor antagonist.
| . | Non-riser pattern . | Riser pattern . | . |
|---|---|---|---|
| Variables . | (n = 310) . | (n = 55) . | P value . |
| Age, years | 67.2 ± 10.6 | 71.9 ± 7.7 | 0.002 |
| Age ≥ 65 years | 215 (69.4) | 47 (85.5) | 0.01 |
| Men, n (%) | 192 (61.9) | 32 (58.2) | 0.60 |
| Body mass index, kg/m2 | 24.8 (3.5) | 23.9 (3.7) | 0.10 |
| Body mass index ≥ 25 kg/m2 | 131 (42.7) | 17 (31.5) | 0.12 |
| Total cholesterol, mmol/L | 5.01 ± 0.87 | 4.83 ± 0.80 | 0.16 |
| Triglycerides, mmol/L | 1.71 ± 1.73 | 1.56 ± 1.18 | 0.57 |
| HDL cholesterol, mmol/L | 1.57 ± 0.45 | 1.45 ± 0.40 | 0.07 |
| LDL cholesterol, mmol/L | 2.84 ± 0.72 | 2.69 ± 0.61 | 0.16 |
| Glucose, mmol/L | 5.92 ± 1.26 | 5.72 ± 1.25 | 0.32 |
| HbA1c, % | 5.7 ± 0.6 | 5.8 ± 0.7 | 0.61 |
| eGFR, ml/min/1.73 m2 | 67.6 ± 15.5 | 63.0 ± 15.8 | 0.06 |
| Chronic kidney disease, n (%) | 82 (26.6) | 20 (37.7) | 0.10 |
| Smoker, n (%) | 139 (47.4) | 26 (50.0) | 0.73 |
| Comorbidities, n (%) | |||
| Dyslipidemia | 253 (82.7) | 47 (87.0) | 0.43 |
| Diabetes mellitus | 81 (26.4) | 11 (20.4) | 0.35 |
| Coronary heart disease | 46 (15.0) | 7 (13.0) | 0.70 |
| Cerebrovascular disease | 28 (9.2) | 10 (18.5) | 0.04 |
| Sleep apnea syndrome | 30 (10.0) | 4 (7.6) | 0.58 |
| Primary aldosteronism | 6 (2.0) | 1 (1.9) | 0.97 |
| Medication use, n (%) | |||
| Calcium channel blockers | 259 (83.6) | 44 (80.0) | 0.52 |
| ARBs | 227 (73.2) | 39 (70.9) | 0.72 |
| ACEIs | 11 (3.6) | 2 (3.6) | 0.97 |
| β-blockers | 48 (15.5) | 8 (14.6) | 0.86 |
| α-blockers | 5 (1.6) | 2 (3.6) | 0.31 |
| Diuretics | 65 (21.0) | 16 (29.1) | 0.18 |
| MRAs | 17 (5.5) | 2 (3.6) | 0.57 |
| Lipid-lowering drugs | 156 (50.3) | 28 (50.9) | 0.94 |
| Hypoglycemic drugs | 48 (15.5) | 8 (14.6) | 0.86 |
| Bedtime administration of antihypertensive drugs | 124 (40.0) | 21 (38.2) | 0.80 |
| Number of antihypertensive drugs | 2.0 ± 0.8 | 2.1 ± 1.0 | 0.90 |
| Office systolic blood pressure, mm Hg | 130.1 ± 16.2 | 130.0 ± 13.8 | 0.93 |
| Office diastolic blood pressure, mm Hg | 72.1 ± 14.0 | 71.7 ± 11.1 | 0.83 |
| Office pulse rate, bpm | 72.3 ± 11.4 | 70.8 ± 12.1 | 0.36 |
| Home blood pressure monitoring | |||
| Nighttime systolic blood pressure, mm Hg | 115.3 ± 11.6 | 129.6 ± 11.6 | <0.001 |
| Nighttime diastolic blood pressure, mm Hg | 68.9 ± 7.8 | 72.5 ± 7.3 | 0.002 |
| Nighttime pulse rate, bpm | 61.3 ± 7.7 | 61.4 ± 8.7 | 0.96 |
| Morning systolic blood pressure, mm Hg | 131.3 ± 13.4 | 128.9 ± 12.4 | 0.21 |
| Morning diastolic blood pressure, mm Hg | 79.2 ± 9.4 | 75.7 ± 10.1 | 0.01 |
| Morning pulse rate, bpm | 66.4 ± 9.3 | 64.4 ± 8.4 | 0.13 |
| Evening systolic blood pressure, mm Hg | 126.0 ± 14.6 | 121.3 ± 12.7 | 0.03 |
| Evening diastolic blood pressure, mm Hg | 73.6 ± 9.6 | 69.1 ± 9.4 | 0.001 |
| Evening pulse rate, bpm | 70.3 ± 9.9 | 69.1 ± 10.8 | 0.43 |
| . | Non-riser pattern . | Riser pattern . | . |
|---|---|---|---|
| Variables . | (n = 310) . | (n = 55) . | P value . |
| Age, years | 67.2 ± 10.6 | 71.9 ± 7.7 | 0.002 |
| Age ≥ 65 years | 215 (69.4) | 47 (85.5) | 0.01 |
| Men, n (%) | 192 (61.9) | 32 (58.2) | 0.60 |
| Body mass index, kg/m2 | 24.8 (3.5) | 23.9 (3.7) | 0.10 |
| Body mass index ≥ 25 kg/m2 | 131 (42.7) | 17 (31.5) | 0.12 |
| Total cholesterol, mmol/L | 5.01 ± 0.87 | 4.83 ± 0.80 | 0.16 |
| Triglycerides, mmol/L | 1.71 ± 1.73 | 1.56 ± 1.18 | 0.57 |
| HDL cholesterol, mmol/L | 1.57 ± 0.45 | 1.45 ± 0.40 | 0.07 |
| LDL cholesterol, mmol/L | 2.84 ± 0.72 | 2.69 ± 0.61 | 0.16 |
| Glucose, mmol/L | 5.92 ± 1.26 | 5.72 ± 1.25 | 0.32 |
| HbA1c, % | 5.7 ± 0.6 | 5.8 ± 0.7 | 0.61 |
| eGFR, ml/min/1.73 m2 | 67.6 ± 15.5 | 63.0 ± 15.8 | 0.06 |
| Chronic kidney disease, n (%) | 82 (26.6) | 20 (37.7) | 0.10 |
| Smoker, n (%) | 139 (47.4) | 26 (50.0) | 0.73 |
| Comorbidities, n (%) | |||
| Dyslipidemia | 253 (82.7) | 47 (87.0) | 0.43 |
| Diabetes mellitus | 81 (26.4) | 11 (20.4) | 0.35 |
| Coronary heart disease | 46 (15.0) | 7 (13.0) | 0.70 |
| Cerebrovascular disease | 28 (9.2) | 10 (18.5) | 0.04 |
| Sleep apnea syndrome | 30 (10.0) | 4 (7.6) | 0.58 |
| Primary aldosteronism | 6 (2.0) | 1 (1.9) | 0.97 |
| Medication use, n (%) | |||
| Calcium channel blockers | 259 (83.6) | 44 (80.0) | 0.52 |
| ARBs | 227 (73.2) | 39 (70.9) | 0.72 |
| ACEIs | 11 (3.6) | 2 (3.6) | 0.97 |
| β-blockers | 48 (15.5) | 8 (14.6) | 0.86 |
| α-blockers | 5 (1.6) | 2 (3.6) | 0.31 |
| Diuretics | 65 (21.0) | 16 (29.1) | 0.18 |
| MRAs | 17 (5.5) | 2 (3.6) | 0.57 |
| Lipid-lowering drugs | 156 (50.3) | 28 (50.9) | 0.94 |
| Hypoglycemic drugs | 48 (15.5) | 8 (14.6) | 0.86 |
| Bedtime administration of antihypertensive drugs | 124 (40.0) | 21 (38.2) | 0.80 |
| Number of antihypertensive drugs | 2.0 ± 0.8 | 2.1 ± 1.0 | 0.90 |
| Office systolic blood pressure, mm Hg | 130.1 ± 16.2 | 130.0 ± 13.8 | 0.93 |
| Office diastolic blood pressure, mm Hg | 72.1 ± 14.0 | 71.7 ± 11.1 | 0.83 |
| Office pulse rate, bpm | 72.3 ± 11.4 | 70.8 ± 12.1 | 0.36 |
| Home blood pressure monitoring | |||
| Nighttime systolic blood pressure, mm Hg | 115.3 ± 11.6 | 129.6 ± 11.6 | <0.001 |
| Nighttime diastolic blood pressure, mm Hg | 68.9 ± 7.8 | 72.5 ± 7.3 | 0.002 |
| Nighttime pulse rate, bpm | 61.3 ± 7.7 | 61.4 ± 8.7 | 0.96 |
| Morning systolic blood pressure, mm Hg | 131.3 ± 13.4 | 128.9 ± 12.4 | 0.21 |
| Morning diastolic blood pressure, mm Hg | 79.2 ± 9.4 | 75.7 ± 10.1 | 0.01 |
| Morning pulse rate, bpm | 66.4 ± 9.3 | 64.4 ± 8.4 | 0.13 |
| Evening systolic blood pressure, mm Hg | 126.0 ± 14.6 | 121.3 ± 12.7 | 0.03 |
| Evening diastolic blood pressure, mm Hg | 73.6 ± 9.6 | 69.1 ± 9.4 | 0.001 |
| Evening pulse rate, bpm | 70.3 ± 9.9 | 69.1 ± 10.8 | 0.43 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; ARB, angiotensin receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; MRA, mineralocorticoid receptor antagonist.
Relationships between the riser pattern and clinical variables
Of the 365 subjects, 55 (15.1%) had a nocturnal riser pattern. The clinical characteristics of subjects based on the riser pattern are summarized in Table 3. Univariate logistic regression analysis showed that age ≥ 65 years (OR, 2.60; 95% CI, 1.18–5.71; P = 0.02) and a history of cerebrovascular disease (OR, 2.26; 95% CI, 1.03–4.97; P = 0.04) were significantly related to a higher risk of the riser pattern and that evening systolic BP of 125–<135 mm Hg (OR, 0.23; 95% CI, 0.09–0.60; P = 0.003) and evening systolic BP of ≥ 135 mm Hg (OR, 0.43; 95% CI, 0.19–0.96; P = 0.04) were significantly related to a lower risk of the riser pattern (Table 4). High-density lipoprotein cholesterol (P = 0.06) and CKD (P = 0.09) had borderline significance. Multivariate logistic regression analysis revealed that age ≥ 65 years (OR, 3.24; 95% CI, 1.27–8.23; P = 0.01) was significantly associated with a higher risk of the riser pattern and that evening systolic BP of 125–<135 mm Hg (OR, 0.22; 95% CI, 0.08–0.58; P = 0.002) and evening systolic BP of ≥ 135 mm Hg (OR, 0.39; 95% CI, 0.16–0.95; P = 0.04) were significantly associated with a lower risk of the riser pattern (Table 4).
Univariate logistic regression analysis and multivariate logistic regression analysis of the relationships between riser pattern and variables
| . | Univariate logistic regression . | Multivariate logistic regression . | ||
|---|---|---|---|---|
| Covariates . | Odds ratio (95% CI) . | P value . | Odds ratio (95% CI) . | P value . |
| Age ≥ 65 years | 2.60 (1.18–5.71) | 0.02 | 3.24 (1.27–8.23) | 0.01 |
| Men | 0.86 (0.48–1.53) | 0.60 | 0.71 (0.36–1.39) | 0.32 |
| Body mass index ≥ 25 | 0.62 (0.33–1.14) | 0.13 | ||
| Total cholesterol, mmol/L | 0.77 (0.54–1.11) | 0.16 | ||
| Triglycerides, mmol/L | 0.93 (0.74–1.18) | 0.54 | ||
| HDL cholesterol, mmol/L | 0.49 (0.23–1.05) | 0.06 | 0.45 (0.20–1.05) | 0.06 |
| LDL cholesterol, mmol/L | 0.73 (0.48–1.13) | 0.15 | ||
| Glucose, mmol/L | 0.87 (0.66–1.15) | 0.30 | ||
| HbA1c, % | 1.13 (0.70–1.84) | 0.62 | ||
| eGFR, ml/min/1.73 m2 | 0.98 (0.96–1.00) | 0.06 | ||
| Chronic kidney disease | 1.67 (0.91–3.07) | 0.09 | 1.17 (0.58–2.33) | 0.66 |
| Smokers | 1.10 (0.61–2.00) | 0.73 | ||
| Dyslipidemia | 1.41 (0.60–3.28) | 0.43 | ||
| Diabetes mellitus | 0.71 (0.35–1.45) | 0.35 | ||
| Coronary heart disease | 0.85 (0.36–1.98) | 0.70 | ||
| Cerebrovascular disease | 2.26 (1.03–4.97) | 0.04 | 1.96 (0.78–4.90) | 0.15 |
| Sleep apnea syndrome | 0.74 (0.25–2.19) | 0.58 | ||
| Primary aldosteronism | 0.96 (0.11–8.18) | 0.97 | ||
| Bedtime dosing of antihypertensive drugs | 0.93 (0.51–1.67) | 0.80 | ||
| Number of antihypertensive drugs | 1.02 (0.73–1.44) | 0.90 | ||
| Office systolic blood pressure < 130 mm Hg | 1 (reference) | |||
| Office systolic blood pressure 130–<140 mm Hg | 1.06 (0.54–2.11) | 0.86 | ||
| Office systolic blood pressure ≥ 140 mm Hg | 0.91 (0.44–1.89) | 0.80 | ||
| Morning systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Morning systolic blood pressure 125–<135 mm Hg | 1.07 (0.55–2.10) | 0.84 | ||
| Morning systolic blood pressure ≥ 135 mm Hg | 0.64 (0.31–1.33) | 0.23 | ||
| Evening systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Evening systolic blood pressure 125–<135 mm Hg | 0.23 (0.09–0.60) | 0.003 | 0.22 (0.08–0.58) | 0.002 |
| Evening systolic blood pressure ≥ 135 mm Hg | 0.43 (0.19–0.96) | 0.04 | 0.39 (0.16–0.95) | 0.04 |
| . | Univariate logistic regression . | Multivariate logistic regression . | ||
|---|---|---|---|---|
| Covariates . | Odds ratio (95% CI) . | P value . | Odds ratio (95% CI) . | P value . |
| Age ≥ 65 years | 2.60 (1.18–5.71) | 0.02 | 3.24 (1.27–8.23) | 0.01 |
| Men | 0.86 (0.48–1.53) | 0.60 | 0.71 (0.36–1.39) | 0.32 |
| Body mass index ≥ 25 | 0.62 (0.33–1.14) | 0.13 | ||
| Total cholesterol, mmol/L | 0.77 (0.54–1.11) | 0.16 | ||
| Triglycerides, mmol/L | 0.93 (0.74–1.18) | 0.54 | ||
| HDL cholesterol, mmol/L | 0.49 (0.23–1.05) | 0.06 | 0.45 (0.20–1.05) | 0.06 |
| LDL cholesterol, mmol/L | 0.73 (0.48–1.13) | 0.15 | ||
| Glucose, mmol/L | 0.87 (0.66–1.15) | 0.30 | ||
| HbA1c, % | 1.13 (0.70–1.84) | 0.62 | ||
| eGFR, ml/min/1.73 m2 | 0.98 (0.96–1.00) | 0.06 | ||
| Chronic kidney disease | 1.67 (0.91–3.07) | 0.09 | 1.17 (0.58–2.33) | 0.66 |
| Smokers | 1.10 (0.61–2.00) | 0.73 | ||
| Dyslipidemia | 1.41 (0.60–3.28) | 0.43 | ||
| Diabetes mellitus | 0.71 (0.35–1.45) | 0.35 | ||
| Coronary heart disease | 0.85 (0.36–1.98) | 0.70 | ||
| Cerebrovascular disease | 2.26 (1.03–4.97) | 0.04 | 1.96 (0.78–4.90) | 0.15 |
| Sleep apnea syndrome | 0.74 (0.25–2.19) | 0.58 | ||
| Primary aldosteronism | 0.96 (0.11–8.18) | 0.97 | ||
| Bedtime dosing of antihypertensive drugs | 0.93 (0.51–1.67) | 0.80 | ||
| Number of antihypertensive drugs | 1.02 (0.73–1.44) | 0.90 | ||
| Office systolic blood pressure < 130 mm Hg | 1 (reference) | |||
| Office systolic blood pressure 130–<140 mm Hg | 1.06 (0.54–2.11) | 0.86 | ||
| Office systolic blood pressure ≥ 140 mm Hg | 0.91 (0.44–1.89) | 0.80 | ||
| Morning systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Morning systolic blood pressure 125–<135 mm Hg | 1.07 (0.55–2.10) | 0.84 | ||
| Morning systolic blood pressure ≥ 135 mm Hg | 0.64 (0.31–1.33) | 0.23 | ||
| Evening systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Evening systolic blood pressure 125–<135 mm Hg | 0.23 (0.09–0.60) | 0.003 | 0.22 (0.08–0.58) | 0.002 |
| Evening systolic blood pressure ≥ 135 mm Hg | 0.43 (0.19–0.96) | 0.04 | 0.39 (0.16–0.95) | 0.04 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate.
Univariate logistic regression analysis and multivariate logistic regression analysis of the relationships between riser pattern and variables
| . | Univariate logistic regression . | Multivariate logistic regression . | ||
|---|---|---|---|---|
| Covariates . | Odds ratio (95% CI) . | P value . | Odds ratio (95% CI) . | P value . |
| Age ≥ 65 years | 2.60 (1.18–5.71) | 0.02 | 3.24 (1.27–8.23) | 0.01 |
| Men | 0.86 (0.48–1.53) | 0.60 | 0.71 (0.36–1.39) | 0.32 |
| Body mass index ≥ 25 | 0.62 (0.33–1.14) | 0.13 | ||
| Total cholesterol, mmol/L | 0.77 (0.54–1.11) | 0.16 | ||
| Triglycerides, mmol/L | 0.93 (0.74–1.18) | 0.54 | ||
| HDL cholesterol, mmol/L | 0.49 (0.23–1.05) | 0.06 | 0.45 (0.20–1.05) | 0.06 |
| LDL cholesterol, mmol/L | 0.73 (0.48–1.13) | 0.15 | ||
| Glucose, mmol/L | 0.87 (0.66–1.15) | 0.30 | ||
| HbA1c, % | 1.13 (0.70–1.84) | 0.62 | ||
| eGFR, ml/min/1.73 m2 | 0.98 (0.96–1.00) | 0.06 | ||
| Chronic kidney disease | 1.67 (0.91–3.07) | 0.09 | 1.17 (0.58–2.33) | 0.66 |
| Smokers | 1.10 (0.61–2.00) | 0.73 | ||
| Dyslipidemia | 1.41 (0.60–3.28) | 0.43 | ||
| Diabetes mellitus | 0.71 (0.35–1.45) | 0.35 | ||
| Coronary heart disease | 0.85 (0.36–1.98) | 0.70 | ||
| Cerebrovascular disease | 2.26 (1.03–4.97) | 0.04 | 1.96 (0.78–4.90) | 0.15 |
| Sleep apnea syndrome | 0.74 (0.25–2.19) | 0.58 | ||
| Primary aldosteronism | 0.96 (0.11–8.18) | 0.97 | ||
| Bedtime dosing of antihypertensive drugs | 0.93 (0.51–1.67) | 0.80 | ||
| Number of antihypertensive drugs | 1.02 (0.73–1.44) | 0.90 | ||
| Office systolic blood pressure < 130 mm Hg | 1 (reference) | |||
| Office systolic blood pressure 130–<140 mm Hg | 1.06 (0.54–2.11) | 0.86 | ||
| Office systolic blood pressure ≥ 140 mm Hg | 0.91 (0.44–1.89) | 0.80 | ||
| Morning systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Morning systolic blood pressure 125–<135 mm Hg | 1.07 (0.55–2.10) | 0.84 | ||
| Morning systolic blood pressure ≥ 135 mm Hg | 0.64 (0.31–1.33) | 0.23 | ||
| Evening systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Evening systolic blood pressure 125–<135 mm Hg | 0.23 (0.09–0.60) | 0.003 | 0.22 (0.08–0.58) | 0.002 |
| Evening systolic blood pressure ≥ 135 mm Hg | 0.43 (0.19–0.96) | 0.04 | 0.39 (0.16–0.95) | 0.04 |
| . | Univariate logistic regression . | Multivariate logistic regression . | ||
|---|---|---|---|---|
| Covariates . | Odds ratio (95% CI) . | P value . | Odds ratio (95% CI) . | P value . |
| Age ≥ 65 years | 2.60 (1.18–5.71) | 0.02 | 3.24 (1.27–8.23) | 0.01 |
| Men | 0.86 (0.48–1.53) | 0.60 | 0.71 (0.36–1.39) | 0.32 |
| Body mass index ≥ 25 | 0.62 (0.33–1.14) | 0.13 | ||
| Total cholesterol, mmol/L | 0.77 (0.54–1.11) | 0.16 | ||
| Triglycerides, mmol/L | 0.93 (0.74–1.18) | 0.54 | ||
| HDL cholesterol, mmol/L | 0.49 (0.23–1.05) | 0.06 | 0.45 (0.20–1.05) | 0.06 |
| LDL cholesterol, mmol/L | 0.73 (0.48–1.13) | 0.15 | ||
| Glucose, mmol/L | 0.87 (0.66–1.15) | 0.30 | ||
| HbA1c, % | 1.13 (0.70–1.84) | 0.62 | ||
| eGFR, ml/min/1.73 m2 | 0.98 (0.96–1.00) | 0.06 | ||
| Chronic kidney disease | 1.67 (0.91–3.07) | 0.09 | 1.17 (0.58–2.33) | 0.66 |
| Smokers | 1.10 (0.61–2.00) | 0.73 | ||
| Dyslipidemia | 1.41 (0.60–3.28) | 0.43 | ||
| Diabetes mellitus | 0.71 (0.35–1.45) | 0.35 | ||
| Coronary heart disease | 0.85 (0.36–1.98) | 0.70 | ||
| Cerebrovascular disease | 2.26 (1.03–4.97) | 0.04 | 1.96 (0.78–4.90) | 0.15 |
| Sleep apnea syndrome | 0.74 (0.25–2.19) | 0.58 | ||
| Primary aldosteronism | 0.96 (0.11–8.18) | 0.97 | ||
| Bedtime dosing of antihypertensive drugs | 0.93 (0.51–1.67) | 0.80 | ||
| Number of antihypertensive drugs | 1.02 (0.73–1.44) | 0.90 | ||
| Office systolic blood pressure < 130 mm Hg | 1 (reference) | |||
| Office systolic blood pressure 130–<140 mm Hg | 1.06 (0.54–2.11) | 0.86 | ||
| Office systolic blood pressure ≥ 140 mm Hg | 0.91 (0.44–1.89) | 0.80 | ||
| Morning systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Morning systolic blood pressure 125–<135 mm Hg | 1.07 (0.55–2.10) | 0.84 | ||
| Morning systolic blood pressure ≥ 135 mm Hg | 0.64 (0.31–1.33) | 0.23 | ||
| Evening systolic blood pressure < 125 mm Hg | 1 (reference) | |||
| Evening systolic blood pressure 125–<135 mm Hg | 0.23 (0.09–0.60) | 0.003 | 0.22 (0.08–0.58) | 0.002 |
| Evening systolic blood pressure ≥ 135 mm Hg | 0.43 (0.19–0.96) | 0.04 | 0.39 (0.16–0.95) | 0.04 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate.
Discussion
In the present study, we investigated the associations of nocturnal hypertension assessed by an HBPM device with clinical variables in patients with hypertension who were treated with antihypertensive drugs. We demonstrated that the diagnostic accuracy of morning systolic BP for subjects with nocturnal hypertension defined as a mean nighttime systolic BP of ≥ 120 mm Hg was superior to that of evening systolic BP and that of office systolic BP. Multivariate analysis revealed that morning systolic BP of 125–<135 mm Hg, morning systolic BP of ≥ 135 mm Hg, and a history of cerebrovascular disease were significantly associated with a higher risk of nocturnal hypertension, whereas bedtime dosing of antihypertensive drugs was significantly associated with a lower risk of nocturnal hypertension. We also demonstrated that age ≥ 65 years was significantly associated with a higher risk of the riser pattern, whereas evening systolic BP of 125–<135 mm Hg and evening systolic BP of ≥ 135 mm Hg were significantly associated with a lower risk of the riser pattern.
In the present study, the diagnostic accuracy of morning systolic BP for subjects with nocturnal hypertension was superior to that of evening systolic BP and that of office systolic BP. Although the precise reasons for the superiority of morning systolic BP to evening systolic BP for predicting subjects with nocturnal hypertension are unknown, evening BP measured before going to bed has been shown to be affected by daytime physical and mental activities and daily activities, including evening meal, bathing, and alcohol consumption,5,23 which may, in part, contribute to the inferiority of evening systolic BP to morning systolic BP for predicting the presence or absence of nocturnal hypertension in medicated patients with hypertension. Asayama et al. reported that HBPM morning hypertension defined as a combination of morning BP of ≥ 135/85 mm Hg and evening BP of < 135/85 mm Hg was a better predictor of stroke than was HBPM evening hypertension defined as a combination of morning BP of < 135/85 mm Hg and evening BP of ≥ 135/85 mm Hg, particularly in patients who were receiving antihypertensive drug treatment,24 indicating that HBPM morning BP is a better predictor than HBPM evening BP of stroke in patients who are being treated with antihypertensive drugs. The results of the present study indicate the possibility that superiority of morning BP to evening BP for predicting stroke events in patients being treated with antihypertensive drugs may, in part, be due to the higher diagnostic accuracy of morning systolic BP for nocturnal hypertension than that of evening systolic BP since nocturnal hypertension assessed by an HBPM device has been shown to be associated with an increased risk of cardiovascular events, especially stroke events.10
The ROC curve of morning systolic BP for predicting subjects with nocturnal hypertension showed that the optimal cutoff value of morning systolic BP to diagnose subjects with nocturnal hypertension was 134.8 mm Hg, suggesting that morning systolic BP should be lowered to at least less than 135 mm Hg, the home systolic BP target previously recommended by the JSH guidelines for the management of hypertension published in 2014, to prevent nocturnal hypertension in medicated patients with hypertension.14 However, the prevalence of nocturnal hypertension was lower in subjects with morning systolic BP of < 125 mm Hg than in subjects with morning systolic BP of 125–<135 mm Hg (13.4% vs. 29.0%). In addition, multivariate analysis revealed that morning systolic BP of 125–<135 mm Hg was significantly associated with a higher risk of nocturnal hypertension when morning systolic BP of < 125 mm Hg was used as a reference. Taken together, the results of the present study support the current home systolic BP target of < 125 mm Hg recommended by the JSH guidelines for the management of hypertension published in 2019.2 The achievement of home morning systolic BP target of < 125 mm Hg may decrease cardiovascular risk through the control of BP throughout 24 hours.
A history of cerebrovascular disease was significantly associated with a higher risk of nocturnal hypertension. It has not been determined whether nocturnal hypertension is the cause or effect of cerebrovascular disease. However, it has been postulated that disturbance in circadian BP rhythm caused by cerebrovascular disease through damage to the circadian system controlled by the clock center in the suprachiasmatic nucleus in the brain may contribute to nocturnal hypertension in subjects with cerebrovascular disease.25,26 Further studies are needed to investigate the mechanisms underlying nocturnal hypertension in patients with cerebrovascular disease.
In the present study, approximately 40% of the subjects took antihypertensive drugs at bedtime and the bedtime dosing of antihypertensive drugs was significantly associated with a lower risk of nocturnal hypertension. Although nighttime BP may be affected by the timing of antihypertensive drug administration, it has not been determined whether bedtime dosing of antihypertensive drugs is more effective than morning dosing of antihypertensive drugs for lowering nighttime BP. Prospective randomized trials have yielded inconsistent results with regard to the usefulness of bedtime dosing of antihypertensive drugs for lowering nighttime BP.27–31 In some of the participants in the present study, the timing of antihypertensive drug administration had probably been adjusted to achieve the home morning BP target before enrollment in this study, which may, in part, contribute to the significant association between bedtime dosing of antihypertensive drugs and lower risk of nocturnal hypertension.
We also investigated the associations between nocturnal riser pattern and clinical variables. Except for evening systolic BP, age ≥ 65 years was the only factor that was significantly associated with a higher risk of the riser pattern assessed by an HBPM device, indicating that it is clinically difficult to predict the presence or absence of the riser pattern in medicated patients with hypertension. Although the riser pattern assessed by ABPM has been shown to be associated with an increased risk of cardiovascular events,32 it has not been determined whether the riser pattern assessed by an HBPM device is associated with an increased risk of cardiovascular events. The method for calculation of the nocturnal dipping level and, consequently, the definition of nocturnal hypertension were different from those in previous studies in which ABPM was used for BP measurements. Further studies are needed to investigate the association between the riser pattern assessed by an HBPM device and cardiovascular events in medicated patients with hypertension.
There are some limitations in this study. First, the cross-sectional design did not allow us to establish a definitive causal relationship between nocturnal hypertension and variables. Second, the results may not be generalizable to other populations since all of the subjects enrolled in this study were Japanese. Third, the possibility of the presence of residual unmeasured confounders cannot be excluded. Fourth, nighttime BP levels are affected by the quality and quantity of nocturnal sleep. However, we did not assess the degree of sleep disturbance caused by cuff inflations during sleep.
In conclusion, the diagnostic accuracy of morning systolic BP for subjects with nocturnal hypertension is superior to that of evening systolic BP and that of office systolic BP in patients with hypertension who are being treated with antihypertensive drugs. Medicated patients with hypertension who have a morning systolic BP of ≥ 125 mm Hg, especially those with a morning systolic BP of ≥ 135 mm Hg, those with a history of cerebrovascular disease, and those who do not take any antihypertensive drugs at bedtime may be at high risk of nocturnal hypertension assessed by an HBPM device.
Appendix Participants and participating centers: Yoshihiko Kinoshita (Kinoshita Clinic), Ryoji Ozono (Ozono Clinic, Internal Medicine and Cardiology), Mitsuaki Nakamaru (Nakamaru Clinic), Masanori Ninomiya (Ninomiya Clinic), Jiro Oiwa (Oiwa Naika), Kotaro Sumii (Mazda Hospital), Narimasa Miho (Mazda Hospital), Takuji Kawagoe (Kawagoe Clinic of Cardiology), Kosuke Takahari (Shobara City Soryo Clinic), Osamu Yoshida (Yoshida Cardiology Clinic), Yasuhiko Hayashi (Tsuchiya General Hospital), Yujiro Ono (Higashi-Hiroshima Medical Center), Hikari Jo (Higashi-Hiroshima Medical Center), Hiroshi Tsushima (Higashi-Hiroshima Medical Center), Yasuo Fukunaga (Fukunaga Cardiology Clinic), Nobuo Shiode (Hiroshima City Hiroshima Citizens Hospital), Hiroki Mitsuda (Mitsuda Clinic), Toshiyuki Matsumoto (Matsumoto Cardiovascular Medical Clinic), Hiroshi Sugino (National Hospital Organization Kure Medical Center and Chugoku Cancer Center), Hironori Ueda (Hiroshima Prefectural Hospital), Naoya Mitsuba (Hiroshima Prefectural Hospital), Hideo Matsuura (Saiseikai Kure Hospital), Koji Kido (Saiseikai Kure Hospital), Kentaro Ueda (Ueda Hatchobori Clinic), Koji Matsumoto (Saiseikai Hiroshima Hospital), Mitsuaki Watanabe (Saiseikai Hiroshima Hospital), Tatsuya Maruhashi (Hiroshima University), Masato Kajikawa (Hiroshima University), Shinji Kishimoto (Hiroshima University), Farina Mohamad Yusoff (Hiroshima University), Kazuaki Chayama (Hiroshima University), Ayumu Nakashima (Hiroshima University), Chikara Goto (Hiroshima International University), Masakazu Takahashi (Yamaguchi University), Yasuki Kihara (Hiroshima University), Yukiko Nakano (Hiroshima University), and Yukihito Higashi (Hiroshima University).
Acknowledgments
We thank Megumi Wakisaka, Miki Kumiji, Ki-ichiro Kawano, and Satoko Michiyama for their excellent secretarial assistance.
Funding
This study was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (18590815 and 21590898 to Y.H.) (16K19408 and 19K17565 to T.M.) and a Grant in Aid of Japanese Arteriosclerosis Prevention Fund (to Y.H.).
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Disclosure
All authors have no conflicts of interests to report.



