-
PDF
- Split View
-
Views
-
Cite
Cite
Mark A Sanders, Paul Muntner, Rong Wei, Daichi Shimbo, Joseph E Schwartz, Lei Qian, C Barrett Bowling, Kimberly Cannavale, Teresa N Harrison, Eva Lustigova, John J Sim, Kristi Reynolds, Comparison of Blood Pressure Measurements from Clinical Practice and a Research Study At Kaiser Permanente Southern California, American Journal of Hypertension, Volume 36, Issue 6, June 2023, Pages 283–286, https://doi.org/10.1093/ajh/hpad020
Close - Share Icon Share
Abstract
Accurate blood pressure (BP) measurement is essential to identify and manage hypertension. Prior studies have reported a difference between BP measured in routine patient care and in research studies. We aimed to investigate the agreement between BP measured in routine care and research-grade BP in Kaiser Permanente Southern California, a large, integrated healthcare system with initiatives to standardize BP measurements during routine patient care visits.
We included adults ≥65 years old with hypertension, taking antihypertensive medication, and participating in the Ambulatory Blood Pressure in Older Adults (AMBROSIA) study in 2019–2021. Clinic BP from routine care visits was extracted from the electronic health record. Research-grade BP was obtained by trained AMBROSIA study staff via an automatic oscillometric device. The mean difference between routine care and research-grade BP, limits of agreement, and correlation were assessed.
We included 309 participants (mean age 75 years; 54% female; 49% non-Hispanic white). Compared with measurements from routine care, mean research-grade systolic BP (SBP) was 0.1 mm Hg higher (95% CI: −1.5 to 1.8) and diastolic BP (DBP) was 0.4 mm Hg lower (95% CI: −1.6 to 0.7). Limits of agreement were −29 to 30 mm Hg for SBP and −21 to 20 mm Hg for DBP. The intraclass correlation coefficient was 0.42 (95% CI: 0.33 to 0.51) for SBP and 0.43 (95% CI: 0.34 to 0.52) for DBP.
High within-person variation and moderate correlation were present between BP measured in routine care and following a research protocol suggesting the importance of standardized measurements.
Accurate blood pressure (BP) measurement is essential for diagnosing hypertension and guiding the management of BP, the world’s leading preventable cause of death and premature disability.1 Prior studies have reported substantial differences between clinic BP measured for patient care compared with research-grade BP measured in research settings with standard protocols.2–8 Most of these studies showed mean clinic BP overestimated mean research-grade systolic BP by as much as 13 mm Hg.3 Measurement errors due to improper technique and lack of standardization have been speculated as a limitation of clinic BP assessed in routine clinical care.9
We investigated the agreement between BP measured in routine patient care and research-grade BP in Kaiser Permanente Southern California (KPSC), a large, integrated healthcare system with longstanding initiatives to standardize clinic BP measurement.10, 11
METHODS
Design, setting, and participants
This cross-sectional study included KPSC members participating in the Ambulatory Blood Pressure in Older Adults (AMBROSIA) prospective cohort study examining the association between BP and fall risk among older adults taking antihypertensive medication. KPSC serves over 4.8 million members. Eligible members were those ≥65 years old with hypertension and taking antihypertensive medication, English-speaking, had health plan membership for ≥24 months, had a clinic systolic BP (SBP) of 110–179 mm Hg and diastolic BP (DBP) of 50–99 mm Hg within 12 weeks before recruitment with no subsequent change in antihypertensive medication and no serious fall injury since last clinic BP assessment. Participants were identified using the KPSC electronic health record. Members with a history of dementia, stroke in the prior 3 months, or residents of skilled nursing or long-term care facilities were ineligible. The study was approved by institutional review boards at KPSC and Columbia University Irving Medical Center, with all participants providing written informed consent.
Data collection
The electronic health record was used to obtain data on participant age, sex, self-reported race/ethnicity, body mass index, smoking, diabetes status, and the current number of antihypertensive medication classes dispensed.
BP measured in routine patient care was obtained from the electronic health record for the most recent non-urgent clinical encounter within 12 weeks before study enrollment. At KPSC, clinic BP is measured using a semi-automatic oscillometric device on the non-dominant arm at the beginning of most encounters. Clinical staff measuring BP are trained under standardized protocols, with regular retraining.10 This involves using patients’ bare arms, correct cuff size, arm supported at heart level, and refraining from talking during measurement. If multiple readings were obtained during a routine care visit or visits on the same day, the average was used in the primary analysis.
Research-grade BP was measured by AMBROSIA study staff during a single visit, following a standardized protocol.12 Participants were seated with their legs uncrossed and their backs supported. The BP cuff was placed on the non-dominant arm with the middle of the cuff at heart level. Participants were instructed to relax and not talk during a 5-minute rest period or during the measurement. BP was measured using a validated automatic oscillometric device (Omron HEM-907XL) programmed to take 3 measurements at 1-minute intervals.13,14 The average of the 3 measurements was used.
Statistical analysis
Participant characteristics were summarized using means with standard deviation (SD) for continuous variables and frequencies for categorical variables. The mean difference between research-grade BP and measurements from routine patient care was assessed using paired t-tests. Intraclass correlation coefficient was used to assess the strength of the relationship between BP obtained in the 2 settings and the Bland-Altman approach was used to assess level of agreement.15 Analyses were conducted separately for SBP and DBP. Sensitivity analyses were conducted using only the first or only the last clinic BP reading when there were multiple readings on the same day. We repeated the analyses restricted to the subgroup of participants with multiple BP measurements from routine care visits on the same day.
RESULTS
Characteristics of the 309 study participants can be found in (Supplementary Table 1 online). Compared with the mean BP from routine patient care, mean research-grade SBP was 0.1 mm Hg higher (95% CI: −1.5 to 1.8) and DBP was 0.4 mm Hg lower (95% CI: −1.6 to 0.7) (Figure 1). The limits of agreement were −29 to + 30 mm Hg for SBP and −21 to + 20 mm Hg for DBP. The Intraclass correlation coefficient was 0.42 (95% CI: 0.33 to 0.51) for SBP and 0.43 (95% CI: 0.34 to 0.52) for DBP.
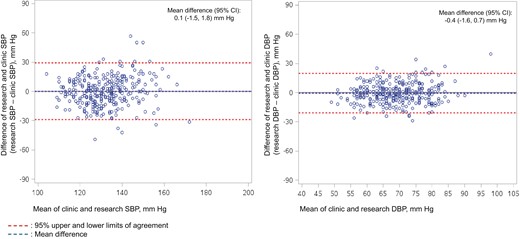
Bland-Altman Plot of the Difference between Research-Grade Blood Pressure and Clinic Blood Pressure and Limits of Agreement. The X-axis is the mean of the 2 blood pressure (BP) assessments (research-grade blood pressure and clinic blood pressure) and the Y-axis is the difference between the 2 assessments. Positive numbers on the Y-axis indicate research-grade blood pressure is higher than clinic blood pressure and negative numbers indicate research-grade blood pressure is lower than clinic blood pressure.
Overall, 42% of participants (n = 129) had multiple BP measurements from routine care visits on the same day. In sensitivity analyses using the first clinic BP, mean research-grade SBP and DBP were lower by 2.7 mm Hg (95% CI: −4.6 to −0.9) and 0.9 mm Hg (95% CI: −2.1 to 0.3), respectively, compared with clinic BP. The limits of agreement were −36 to + 30 mm Hg for SBP and −22 to + 20 mm Hg for DBP. When using the last clinic BP measurement, mean research-grade SBP was 2.8 mm Hg higher (95% CI: 1.1 to 4.5) and DBP was 0.1 mm Hg lower (95% CI: −1.3 to 1.1) compared with clinic BP. The limits of agreement were −27 to + 33 mm Hg for SBP and −21 to + 21 mm Hg for DBP.
The mean difference, limit of agreement, and Intraclass correlation coefficient between research-grade BP and measurements from routine patient care among the subgroup of 129 participants with multiple clinic BP measurements on the same day are presented in (Supplementary Table 2 online). Compared with mean BP from routine patient care, mean research-grade SBP and DBP were lower by 2.6 mm Hg (95% CI: −5.4 to 0.3) and 1.4 mm Hg (95% CI: −3.3 to 0.4), respectively. The limits of agreement were −35 to + 30 mm Hg for SBP and −22 to + 19 mm Hg for DBP. The Intraclass correlation coefficient was 0.39 (95% CI: 0.24 to 0.53) for SBP and 0.50 (95% CI: 0.36 to 0.62) for DBP. Using the first clinic BP, mean research-grade SBP and DBP were lower by 9.4 mm Hg (95% CI: −13.0 to −6.2) and 2.6 mm Hg (95% CI: −4.5 to −0.6), respectively, compared with clinic BP. The limits of agreement were −46 to + 27 mm Hg for SBP and −25 to + 20 mm Hg for DBP. When using the last clinic BP measurement, mean research-grade SBP was 3.9 mm Hg higher (95% CI: 0.9 to 6.9) and DBP was 0.7 mm Hg lower (95% CI: −2.7 to 1.3) compared with clinic BP. The limits of agreement were −31 to + 39 mm Hg for SBP and −23 to + 22 mm Hg for DBP.
DISCUSSION
At the population level, mean BP measured in a research study and clinical practice were approximately equivalent. At the individual level, within-person differences between the measures were often substantial with wide limits of agreement (up to 30 mm Hg for SBP and 20 mm Hg for DBP) and had only moderate correlation.9 While wide individual-level variation and moderate correlation are consistent with previous studies, the close agreement between measures at the population level is notable.2–8
Prior studies have compared clinic and research-grade BP with most reporting substantial mean differences, with clinic BP overestimating research-grade SBP by 4 to 13 mm Hg and DBP by 2 to 7 mm Hg.2–5,7,8 Clinic SBP was 13 mm Hg higher compared to research-grade SBP among VA patients with treated hypertension.3 Drawz and colleagues found a 7 mm Hg higher clinic SBP when measured following the research protocol for the Systolic Blood Pressure Intervention Trial (SPRINT), compared with measurements obtained in clinical practice.2 In other studies, SBP was under-estimated by 4 mm Hg and DBP by 3 mm Hg when measured in a clinical practice compared to a research study among primarily overweight/obese women.6
The approximate equivalence between clinic and research-grade BP at the population level in this study may signal more accurate BP measurement technique in the health system. KPSC implemented initiatives to standardize BP measurements in routine clinical practice, including a 4-step protocol that reduced measurement error by 40%.11 Therefore, the current findings suggest that population-level agreement may be useful for generalized, system-wide monitoring of BP or inform large-scale initiatives to standardize BP measurement. However, application at the individual level is less reliable given the high within-person variation and only moderate correlation. Clinicians should be prepared to identify situations where clinic BP is likely inaccurate and consider other measurement methods (i.e., home or ambulatory monitoring) or assess BP over multiple visits before making treatment decisions.
Several limitations of the current study should be considered. BP from routine patient care and research BP measurements did not occur on the same day; the mean (SD) number of days between clinic BP and research-grade BP was 35.4.22.2 BP varies from visit to visit, which may be explained by situational factors such as measurement technique, white coat effect, and recency of exercise or caffeine.9 The different devices used to measure clinic and research-grade BP may have contributed to discrepancies.16 These results from a single, integrated healthcare system with standardized procedures to measure BP during routine patient care may not represent other clinic settings. While KPSC implemented BP standardization initiatives, the ability of the initiatives to estimate research grade BP estimates has not been previously reported.
Among older adults with treated hypertension in an integrated healthcare system with initiatives to standardize BP measurement, high within-person variation and only moderate correlation between measures obtained in routine care and following a research protocol is consistent with previous studies. These findings suggest the importance of measuring BP over multiple clinic visits following a standardized protocol.
Supplementary MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
FUNDING
This research was supported by R01 HL136445 from the National Heart, Lung and Blood Institute.
DISCLOSURE
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
MAS contributed to the study design and development of the research question and drafted the manuscript for the current analysis. RW and LQ conducted analyses, interpreted findings, and contributed to the preparation of the manuscript. PM, DS, JES, CBB, KLC, TNH, EL, JJS, and KR led the data collection, data access, study design, interpretation of findings, and manuscript preparation. All co-authors contributed to the manuscript and approved this submission.
DATA AVAILABILITY
Anonymized data that support the findings of this study may be made available from the investigative team in the following conditions: (1) agreement to collaborate with the study team on all publications, (2) provision of external funding for administrative and investigator time necessary for this collaboration, (3) demonstration that the external investigative team is qualified and has documented evidence of training for human subjects protections, and (4) agreement to abide by the terms outlined in data use agreements between institutions.


