-
PDF
- Split View
-
Views
-
Cite
Cite
Shamim Shahi, Sandra L Jackson, Taylor E Streeter, Siran He, Hilary K Wall, Cuff Size Variation Across Manufacturers of Home Blood Pressure Devices: A Current Patient Dilemma, American Journal of Hypertension, Volume 36, Issue 10, October 2023, Pages 532–535, https://doi.org/10.1093/ajh/hpad060
Close - Share Icon Share
Abstract
The American Heart Association (AHA) recommends cuff sizes of blood pressure (BP) monitoring devices based on patient arm circumference, which is critical for accurate BP measurement. This study aimed to assess cuff size variation across validated BP devices and to examine the degree of alignment with the AHA recommendations.
Data on home BP devices were obtained from the US BP Validated Device Listing website and listed cuff sizes were compared against AHA recommendations: small adult (22–26 cm), adult (27–34 cm), large (35–44 cm), and extra-large (XL) (45–52 cm).
There were 42 home validated BP devices from 13 manufacturers, and none offered cuffs that were aligned with the AHA recommendations. Over half of the devices (22, 52.4%) were compatible with only a broad-range cuff, generally excluding arm sizes larger than 44 cm. Only 5 devices from 4 manufacturers offered a cuff labeled “XL,” and of these, only 3 devices had sizes that covered the AHA XL range. Terminology lacked consistency with manufacturers using: different labels to describe the same-sized cuffs (e.g., 22–42 cm was labeled “integrated,” “standard,” “adult,” “large,” and “wide range”); the same labels to describe differently sized cuffs (e.g., cuffs labeled “large” were sized 22–42 cm, 32–38 cm, 32–42 cm, 36–45 cm).
Manufacturers of US home BP devices employ inconsistent terminologies and thresholds for cuff sizes, and sizes were not aligned with AHA recommendations. This lack of standardization could pose challenges for clinicians and patients attempting to select a properly sized cuff to support hypertension diagnosis and management.
Hypertension is a major public health issue affecting about half of adults in the United States.1 It is a major risk factor for heart disease and stroke, which are the first and fifth leading causes of death, respectively.2 Uncontrolled blood pressure (BP) also contributes to conditions such as kidney disease, heart failure, and dementia.3 Hypertension can be managed through early diagnosis, regular monitoring, dietary and lifestyle modification, and pharmacologic treatment.4 Self-measured blood pressure monitoring (SMBP), with clinical support, has been shown to be an effective strategy for managing hypertension.5
For effective SMBP, accurate BP measurement depends on factors including the clinical validity of the device and appropriate cuff size selection.5 There has been considerable progress toward validating BP devices; the US Blood Pressure Validated Device Listing (VDL) was created in April 2020.6,7 Using a cuff that is too tight or too loose can lead to inaccurate BP readings.8 Ensuring proper fit requires clinicians to measure a patient’s arm circumference and encourage the purchase of a properly sized cuff according to the American Heart Association (AHA) sizing recommendations (e.g., large adult).9,10 However, it is uncertain whether manufacturer sizes are consistent with recommended sizing, potentially leading to inaccurate measurements.
No studies, to date, have examined the BP cuff size and naming variations across devices included on the VDL. The goal of this study was to assess the standardization of available cuffs for home BP devices listed on the VDL and to examine the degree to which cuff size ranges are aligned with the AHA sizing recommendations.
METHODS
We extracted data on home BP measurement devices from the VDL, maintained by the National Opinion Research Center (NORC) at the University of Chicago on behalf of the American Medical Association (AMA); data are publicly available at https://www.validatebp.org/.6 NORC conducts an independent review by clinicians to ensure that BP devices included in the VDL meet the validation criteria for clinical accuracy using internationally accepted protocols.6 Data for all available validated home BP devices were extracted from the VDL as of March 2023.6 For each device, we extracted the brand, device name, model number, and available BP cuff size(s). Manufacturer cuff sizes were compared against the AHA-recommended BP cuff size categories: small adult (22–26 cm), adult (27–34 cm), large adult (35–44 cm), and extra-large (XL) adult (45–52 cm).9,10 Data were analyzed descriptively, and visualizations were used to compare the cuff sizes of BP devices within and across different manufacturers.
RESULTS
Overall, the VDL included 42 devices from 13 manufacturers (Figure 1). Although AHA recommends four categories to classify BP device cuff sizes (small, adult, large, and XL), many manufacturers offered a broad-range cuff that did not fit into any of the AHA standard sizes but rather spanned multiple AHA size categories. Manufacturers used different size terminologies, such as “wide range,” “standard,” “integrated,” “smooth fit,” and “adult” to describe this category. Of all 42 devices, 32 devices offered a broad-range cuff. Of these, 22 devices offered a broad-range cuff only, while the other 10 were also available in additional sizes. The most common size of broad-range cuff was 22–42 cm (28 of 42 total devices, 66.7%; 20 of 22 devices with a broad-range only, 90.9%).
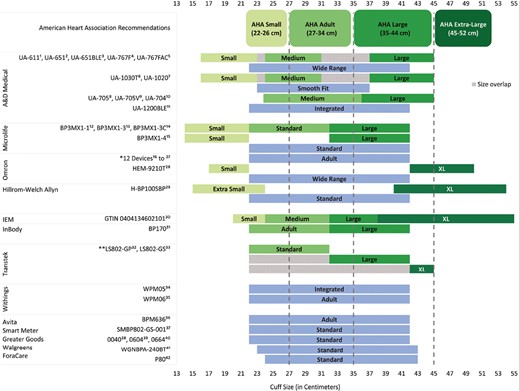
Cuff sizes of 42 home-based devices from 13 manufacturers on the US BP Validated Device Listing and comparison with the American Heart Association (AHA) recommendations. Devices from the same manufacturer with similar cuff size options and terminologies were grouped together and sequential numbers were used to indicate each device. *Omron 12 devices: HEM-9200T, BP7000, BP7250 HEM-7311, BP7200 HEM-7311, BP7100 HEM-7311, BP5450 HEM-7311, BP5350 HEM-7311, BP7450 HEM-7320, BP7350 HEM-7320, BP5100 HEM-7311, BP5250 HEM-7320, BP7900-HEM7311. **Transtek standard size (22–32 cm) fully overlaps with the ranges for its large (22–42 cm) and XL (22–45 cm) sizes.
Of all 42 devices, 20 (47.6%) offered multiple cuff sizes. Of these, 14, 17, 18, and 3 devices covered at least part of AHA small, adult, large, and XL cuff size ranges, respectively. However, cuff sizes did not align exactly with the AHA size ranges. Different manufacturers had varying thresholds for these sizes, and some used different terminologies to refer to similar sizes.
The AHA recommendation for a small cuff size is 22–26 cm. The upper and lower limits for small cuff sizes varied across manufacturers, and no cuffs had an upper size limit of 26 cm. Among devices produced by A&D Medical, the range for “small” was 16–24 cm. Devices by Hillrom-Welch Allyn did not have a “small” cuff but had an “extra small” cuff with a similar range (15–24 cm). The remaining manufacturers with cuffs labeled “small”—IEM, Omron, and Microlife had ranges of 20–24 cm, 17–22 cm, and 14–22 cm, respectively.
The AHA recommendation for an adult cuff size is 27–34 cm, but again, no cuff sizes matched this range, and most ranges were broader. For example, most A&D Medical devices offered a “medium” cuff of 23–37 cm, except one device with a narrower range (UA705, 23.8–36 cm). Microlife, InBody, and Transtek each had an available cuff size of 22–32 cm, though they used different terminologies (Microlife and Transtek labeled this “standard,” while InBody labeled it “adult”).
The AHA recommendation for the large cuff size range is 35–44 cm, but no cuff sizes matched this range, and there was substantial variation in “large” cuffs. Transtek had the widest range (22–42 cm), and IEM the narrowest (32–38 cm). Only A&D had a range that covered the upper bound of an AHA large-size cuff (44 cm), with a range of 31–45 cm.
The AHA recommendation for an XL cuff is 45–52 cm. Only 5 devices, from 4 manufacturers, offered an “XL” cuff. The range for “XL” by Transtek was 22–45 cm, which is below the AHA-recommended XL range. The remaining three devices had ranges that overlapped with the AHA XL size range (38–55 cm for IEM, 40–54 cm for Hillrom-Welch Allyn, and 42–50 cm for Omron); only two devices fully covered the upper bound of the AHA range (52 cm).
As seen in Figure 1, overlapping sizes were an issue, even within manufacturers. For example, the “medium” and “large” cuff sizes within some A&D Medical devices had an area of overlap from 32 to 36 cm. In addition, the Transtek “standard” cuff (22–32 cm) fully overlaps with the ranges for its “large” (22–42 cm) and “XL” (22–45 cm) sizes.
DISCUSSION
In this first-ever, comprehensive assessment of home BP device cuff sizes across manufacturers listed on the VDL, we observed that the manufacturers used different terminologies and thresholds for cuff sizes, and no size ranges aligned exactly with the AHA recommendations. Many available sizes might not fit patients appropriately, potentially leading to inaccurate BP measurement. For example, very few available devices covered AHA’s XL size range, while over half of devices offered only a broad-range size that may not be appropriate for smaller arms and may not cover larger arms. Additionally, cuff sizes overlapped within manufacturers, which could create confusion among patients seeking an appropriate cuff size.
There were numerous discrepancies in cuff size terminology, which could lead to difficulties for patients attempting to select a properly sized cuff. First, none of the manufacturers on the VDL matched the AHA-recommended size ranges exactly, even when manufacturers used the same size terms (e.g., “adult”). Therefore, if a clinician measures a patient’s arm circumference at 30 cm and recommends the patient purchase an adult-sized cuff, it’s possible that the patient may purchase an incorrectly sized “adult” cuff. Second, size ranges also lacked consistency across manufacturers: the same labels were used to refer to different size ranges by different manufacturers. Therefore, if a patient determines based on her arm circumference that she needs a size “large” from one manufacturer, she cannot assume that she would also fit a size “large” from any other manufacturer (or even, in some cases, from a different device by the same manufacturer). Third, manufacturers frequently used different labels to refer to cuffs that would fit the same-sized arm. For example, a patient with an arm circumference of 23 cm would fit within the listed size ranges for cuffs labeled “small,” “medium,” “wide range,” “smooth fit,” “integrated,” “standard,” “adult,” “large,” and “XL” across various manufacturers. These discrepancies in terminologies could make it challenging for clinicians to instruct patients about purchasing the proper size; currently, clinicians must provide the actual arm circumference and not cuff size terminology (such as “large” cuff).
In addition to terminology discrepancies, there were substantial areas of overlap across different cuff sizes, even by the same manufacturer. This could create confusion among patients when determining the appropriate cuff size, given a particular arm circumference. For example, a patient with an arm circumference of 32 cm who purchases a Transtek device would not know whether to select the “standard” (22–32 cm), “large” (22–42 cm), or “XL” (22–45 cm) size for a Transtek device due to the overlap across all three cuff sizes.
Many manufacturers have devices with only a broad-range cuff. Manufacturers used various terms to refer to this one-size-fits-all range, such as “wide range,” “smooth fit,” “adult,” and “standard,” which could lead to confusion. These devices do not encompass the full large and XL ranges recommended by AHA, and, hence, may not accommodate individuals with obesity. Additionally, the cuff could fit too loosely for persons who need an AHA small cuff, even though the small range is encompassed in the broad-range size. This could lead to falsely low BP readings.11 There is some evidence that using a broad-range cuff could lead to misdiagnosis and mistreatment of hypertension.12 Given the diversity of arm circumferences in the population, a one-size-fits-all approach may not be ideal to meet individual patients’ needs.
Despite the large number of validated devices, relatively few cuffs on the VDL covered the full AHA range for XL. A recent study using National Health and Nutrition Examination Survey (NHANES) 2015–2020 data reported that about 59% of men with hypertension and 42% of women with hypertension required a large or XL BP cuff.13 Only devices labeled “large” from one manufacturer (A&D) covered the full range of the AHA large size, and only two devices covered the full AHA XL range. The scarcity of large and XL cuffs is concerning, given the increasing prevalence of obesity in the United States,14 and the fact that persons with obesity are at higher risk of hypertension.15 In addition, as non-Hispanic Black adults are at higher risk of obesity, lack of large and XL cuffs could contribute to existing disparities in hypertension management.16 One older study showed that clinicians were most likely to use incorrect cuff sizes among patients who needed large cuffs, compared with other cuff sizes.17 Additionally, a recent study showed that current devices do not adequately accommodate patients with obesity due to their large arm circumference and unique conical shape, and persons with obesity reported unpleasant experiences such as pain and skin damage.18 Using cuffs that are too tight for persons with obesity could potentially result in falsely high BP readings, and there is evidence that the risk of hypertension has been overestimated in persons with obesity due to the use of too-small cuffs.19
Our study has several limitations. First, the differences in cuff sizes were examined only descriptively, and evaluating the accuracy of differing cuff size ranges regarding BP measurements on various arm sizes was beyond the scope of this study. Second, results were restricted to home BP monitoring devices, and findings cannot be extrapolated to other VDL device types such as ambulatory, kiosk, office, office automated, remote patient monitoring, or wrist. Third, we assessed cuff size ranges and terminologies on devices only from the VDL; results are not generalizable to all devices sold in the United States.
In conclusion, manufacturers of home BP devices on the VDL employ inconsistent terminologies and cuff size ranges, which do not align with AHA recommendations. Discrepancies in cuff sizes and terminologies could lead to patient and clinician confusion and inaccurate BP readings. Using standardized terms and cuff sizes could reduce errors in BP measurements, allowing patients to better understand their BP and potentially improving the accuracy of hypertension diagnosis and management. These findings could inform decisions by policymakers, regulatory bodies, and manufacturers regarding standardizing cuff sizes and terminology. Standardized sizes could better support clinicians in recommending properly sized cuffs to their patients and could better support patients in selecting an appropriate SMBP device and cuff.
Conflict of Interest
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors have no conflicts of interest to disclose.


