-
PDF
- Split View
-
Views
-
Cite
Cite
Sambit K Mohanty, Sourav K Mishra, Ankit Tiwari, Shivani Sharma, Mohit Bhardwaj, Niharika Pattnaik, Sunil Jaiswal, Manas R Baisakh, Subodh Das, Manas R Pradhan, Tapas R Swain, Kaliprasad Satpathy, Sean R Williamson, Anil V Parwani, Reappraisal of HER2 Amplification in High-Grade Urothelial Carcinoma Based on 2018 ASCO/CAP Clinical Practice Guidelines, American Journal of Clinical Pathology, Volume 156, Issue 6, December 2021, Pages 1130–1141, https://doi.org/10.1093/ajcp/aqab083
Close - Share Icon Share
Abstract
To examine and compare human epidermal growth factor receptor 2 (HER2) amplification status in high-grade urothelial carcinoma (HGUCa), using both 2013 and 2018 HER2 reporting guidelines for breast carcinoma from the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP).
HER2 status by fluorescence in situ hybridization (FISH) assay in 78 cases of HGUCa was compared using 2013 and 2018 HER2 reporting guidelines.
HER2 amplification was observed in 22 (28.2%) of 78 tumors, of which 17 were in group 1, 1 in group 2, and 2 each in groups 3 and 4 (FISH assay, 2018). The remaining 14 HER2-amplified tumors (FISH assay, 2013) became negative, falling into group 2 (FISH assay, 2018) and were either negative or equivocal on immunohistochemistry (IHC, 2018). All FISH-negative tumors (n = 37) using 2013 criteria remained negative (group 5, 2018). FISH-equivocal tumors (2013) were further categorized into HER2 amplified (n = 1) and HER2 negative (n = 4) (2018). Overall, 20 (25.6%) tumors had discordant HER2 FISH results (2018 vs 2013).
Implementing 2018 guidelines, HER2 amplification decreased from 36 to 22 cases. The group with a HER2/CEP17 ratio of 2 or more and average HER2 copy number less than 4 (group 2) were predominantly negative by IHC, suggesting a biologically distinct group of HGUCa that is different from HER2-amplified tumors, which may not respond to HER2-targeted therapy.
Currently, there is a lack of literature on appropriate validation and standardized implementation of HER2 reporting in high-grade urothelial carcinoma (HGUCa) of the urinary bladder.
Implementing 2018 guidelines, HER2 amplification decreased from 36 (2013) to 22 (2018) cases of HGUCa.
The group with a HER2/CEP17 ratio of 2 or more and average HER2 copy number less than 4 (group 2) were predominantly negative by immunohistochemistry and hence HER2 nonamplified, which may not respond to HER2-targeted therapy.
The human epidermal growth factor receptor 2 (HER2) is encoded by the HER2 proto-oncogene located on chromosome 17q21. HER2 belongs to the class I receptor tyrosine kinase transmembrane proteins and is responsible for tumor cell proliferation, migration, and invasion, all hallmarks of cancer.1,2HER2 amplification is observed in 15% to 30% of invasive breast carcinomas, 10% to 30% of gastric adenocarcinomas, up to 83% of esophageal adenocarcinomas (including gastroesophageal adenocarcinomas), 14% to 80% of uterine serous carcinomas, 20% to 30% of ovarian cancers, and up to 80% of cases of bladder urothelial carcinoma (UCa). HER2 is amplified in 12% and 7% and mutated in 3% and 7% of pulmonary and colonic adenocarcinomas, respectively.3-6
Previous studies have demonstrated HER2 overexpression in 17% to 76% of cases of noninvasive UCa and 23% to 80% of invasive carcinomas.7 Also, the Cancer Genome Atlas Research Network reported up to 9% HER2 gene mutation or amplification in UCa.8 In the largest analysis on HER2 status in UCa, Laé et al9 reported that 9% of their UCa cases exhibited either an equivocal (2+) or positive (3+) staining for HER2 using the 2007 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines. When those cases were analyzed using fluorescence in situ hybridization (FISH) assay, 5.1% of the tumors were HER2 amplified, and they were of the HER2 3+ types.9 These observations attest to the fact that true HER2 overexpression occurs in UCa by amplification as in breast cancer.8-10 More contemporary studies demonstrated HER2 overexpression in UCa in the range of 4.3% to 31.12%.11-14 HER2 overexpression in UCa is not uniform across all histologies, being more frequent in micropapillary (MP) carcinomas (12%), UCa with squamous differentiation (11%), and sarcomatoid carcinoma (9%) than in conventional UCa (6%).15,16 HER2 overexpression has been correlated with higher grade and higher T stage, and it is an independent predictor of disease progression and survival in UCa.11-13,15 Therefore, these salient observations make HER2 testing and reporting crucial in bladder cancers, as both a prognostic and a predictive biomarker for response to anti-HER2 therapy. However, results from the clinical trials with anti-HER2 therapy in bladder cancer have been disappointing at best, with minimal clinical benefit.17,18 One of the reasons for the discrepancy between HER2 overexpression and clinical benefit could be due to inclusion of HER2 1+ (negative), 2+ (equivocal) cases and an improper cutoff value for FISH (positive or negative) assays in these trials.18
A few clinical trials on trastuzumab (an anti-HER2 monoclonocal antibody) in localized and advanced UCa have included tumors with HER2 immunohistochemistry (IHC) 2+.19,20 Such patients are unlikely to benefit from anti-HER2 therapy, as has been documented in the breast cancer. Another well-designed phase 2 randomized controlled trial including tumors that are HER2 IHC 2+ or 3+ with HER2 amplification by FISH failed to demonstrate any benefit of adding trastuzumab. However, a HER2/CEP17 ratio of more than 2 was the only criterion used to define HER2 amplification. Also, there was no mention about the average HER2 copy number in these cases (<4 vs ≥4 HER2 signals/cell). The authors concluded that the unexpectedly low incidence of HER2 amplification precluded the detection of a significant difference in efficacy on the addition of trastuzumab to chemotherapy.21 A few other studies have also failed to demonstrate meaningful benefit of adding HER2 tyrosine kinase inhibitors such as lapatinib, afatinib, and neratinib in advanced UCa. In these studies, tumors with either epidermal growth factor receptor or HER2 IHC positivity or HER2/HER3 mutations were included.22-24
IHC and FISH are the two principal modalities for HER2 testing. IHC 1+ and 2+ (with negative FISH result) do not benefit from anti-HER2 therapy.25 Thus, HER2 3+ using IHC or FISH positivity/amplification is the prerequisite for anti-HER2 therapy. Consequently, the ASCO/CAP have established guidelines for HER2 reporting in the breast cancer to bring uniformity in HER2 reporting.10,26-28 These guidelines have been extrapolated for HER2 reporting in other malignancies. The first ASCO/CAP guidelines for HER2 reporting in breast cancer were established in 2007.26 Subsequently, there was a revision in 2013 and a focused update in 2018.10,27,28 Per 2007 guidelines, the cutoff for HER2 positivity using IHC was 30% or more, thus missing the true HER2-positive cases (high false negative).26 To overcome this, the 2013 ASCO/CAP guidelines cutoff for HER2 positivity was kept at more than 10%, which was also the inclusion criterion in the pivotal adjuvant trastuzumab trials. The 2013 guidelines also emphasized the need to do tests in CAP-accredited laboratories and periodic competency assessment in these laboratories.10,27 The 2018 ASCO/CAP guidelines for HER2 testing and reporting have ushered in a substantial degree of clarity and reproducibility in HER2 reporting.10,27,28
Liu et al29 retrospectively assessed the impact of the revised 2018 ASCO/CAP guidelines on 2,233 cases of invasive breast cancer. Compared with the 2013 guidelines, HER2 status in 183 (8.2%) cases was redefined when assessed by the 2018 guidelines. Among these 183 cases, 175 FISH-equivocal cases according to the 2013 guidelines were redefined as HER2 nonamplified (n = 173) or HER2 amplified (n = 2). Eight previously classified as HER2-amplified cases were reported to be nonamplified in the 2018 scheme, all of which had HER2 IHC scores of 1+ or 2+. The reassessment completely eliminated the HER2 FISH-equivocal subset per the 2013 guidelines. Almost all cases (173/175) classified as HER2 FISH equivocal were reclassified as nonamplified per the 2018 guidelines (19 IHC 0/1+ and the remaining IHC 2+ cases on recounting generated similar HER2 FISH results).29 Overall, using the 2018 ASCO/CAP HER2 reporting guidelines in breast cancer, 4.6% to 16% HER2 FISH results have been reclassified, and this discordance has been driven by an increase in HER2 FISH negativity by 4% to 15.5%. At the same time, the reported positivity rate decreased by 0.4% to 1.1%.29-31
Currently, there is a lack of literature on appropriate validation and standardized implementation of HER2 reporting in high-grade UCa (HGUCa) of the urinary bladder. Also, there are no data comparing HER2 reporting per the 2013 guidelines with the 2018 ASCO/CAP guidelines in HGUCa. This study aims at examining the frequency of HER2 amplification in HGUCa, using both the 2013 and 2018 HER2 reporting guidelines and the utility of the 2018 scoring criteria over the 2013 scoring system to appropriately delineate the cases that fall into the equivocal and borderline categories and where either unnecessary targeted therapy can be avoided (true negative) or can be correctly identified as positives who can benefit from anti-HER2 therapy.
Materials and Methods
Patients and Case Selection
After institutional review board approval, a total of 78 consecutive tumors from 78 individual patients with HGUCa were collected from the anatomic pathology archive of the Advanced Medical Research Institute. The demographics (age and sex) and relevant clinical parameters (type of specimen and clinical stage), as well as results of various IHC stains to evaluate the molecular subtypes (luminal subtype: GATA3, cytokeratin [CK] 20, estrogen receptor, and uroplakin II; basal: CK5/6, CK14, and CD44; nonluminal and nonbasal or a basoluminal subtype with luminal as well as basal marker expression), were recorded. The H&E-stained slides were reviewed to confirm the diagnosis and subtype the tumor. Finally, the histologic and molecular subtypes and pathologic stage of the individual cases were tabulated.
HER2 Immunohistochemistry
The 4-µm sections were made of the formalin-fixed, paraffin-embedded tissues. The preanalytical parameters involved were as follows: all tumor specimens were obtained by transurethral resection of the bladder tumor. Cold ischemia time (time between tissue removal and initiation of fixation) ranged from immediate fixation to 10 minutes (<30 minutes is the ideal cold ischemia time), and 10% neutral buffered formalin was used in all tumors as the fixative. The total fixation ranged between 6 and 24 hours. None of the specimen was decalcified, and the antibody clone used was approved by the US Food and Drug Administration. IHC for HER2 was performed in an automated Ventana IHC Platform (Ventana Medical Systems). IHC was performed using HER2 antibody (anti-HER2/neu [4B5] rabbit monoclonal primary antibody) as per the manufacturer’s instructions using appropriate positive and negative controls. HER2 staining was validated in 20 known positive and 20 known negative controls in different batches as the validation set prior to implementing the tests in this cohort of HGUCa. Ultra Cell Conditioner 1 (Ventana Medical Systems) was used at a high pH for 64 minutes for antigen retrieval, the incubation time for the primary antibody was 32 minutes, and the UltraView Universal DAB detection kit (Ventana Medical Systems) was used. The scoring for HER2 was done by the staff pathologists per ASCO/CAP guidelines 2013/2018, as described below.
The following guidelines were used to assess HER2 staining in each tumor. Per 2013 ASCO/CAP guidelines, the IHC scoring criteria are as follows: score 0, no staining or faint incomplete membrane staining in not more than 10% of the invasive carcinoma cells; score 1+, weak and incomplete membrane staining in more than 10% of the invasive carcinoma cells; score 2+, complete intense membrane staining in not more than 10% of the invasive carcinoma cells or weak/moderate heterogeneous incomplete staining in more than 10% of the invasive carcinoma cells; and score 3+, strong complete homogeneous membrane staining in more than 10% of the invasive carcinoma cells.27 Per 2018 ASCO/CAP guidelines, the IHC scoring criteria are as follows: score 0, no staining observed or membrane stating that is incomplete and faint/barely perceptible and within 10% or less of tumor cells; score 1, incomplete membrane staining that is faint/barely perceptible and within more than 10% of tumor cells; score 2, weak to moderate complete membrane staining in more than 10% of tumor cells or complete membrane staining that is intense but within 10% or less of tumor cells; and score 3, complete membrane staining that is intense and more than 10% of tumor cells (readily appreciated using a low-power objective and observed within a homogeneous and contiguous population of invasive tumor cells).28
HER2 FISH
H&E-stained slides were reviewed by the staff pathologists to identify the invasive urothelial carcinoma component. Following this, 4-µm-thick sections of tumor tissues were prepared for FISH analysis. The dual-color HER2/CEP17 assay (Abbot Molecular) was performed for FISH analysis. Dual-fluorescent hybridization signals of HER2/CEP17 were analyzed and captured by Bioview software. HER2 and CEP17 signals were scored manually by the staff pathologists for 200 nuclei. Calculations of HER2/CEP17 were made independently, and the average was used to determine the final copy number. Results were interpreted by pathologists per the ASCO/CAP guidelines 2013 and 2018.27,28
2013 and 2018 HER2 FISH Scoring Method
Per 2013 ASCO/CAP guidelines, a positive test met the following criteria: HER2/CEP17 ratio of 2.0 or more with an average HER2 copy number of 4.0 or more signals per cell, HER2/CEP17 ratio of 2.0 or more with an average HER2 copy number of less than 4.0 signals/cell, or HER2/CEP17 ratio less than 2.0 with an average HER2 copy number of 6.0 or more signals/cell. FISH-equivocal result was based on a HER2/CEP17 ratio less than 2.0 with an average HER2 copy number of 4.0 or more and less than 6.0 signals/cell. FISH-negative result was based on a HER2/CEP17 ratio of less than 2.0 with an average HER2 copy number of less than 4.0 signals/cell.27
According to the 2018 ASCO/CAP guidelines, primarily the tumors were graded into five principal groups: group 1, HER2/CEP17 ratio of 2.0 or more and 4.0 or more HER2 signals/cell; group 2, HER2/CEP17 ratio of 2.0 or more and less than 4.0 HER2 signals/cell; group 3, HER2/CEP17 ratio of less than 2.0 and 6.0 or more HER2 signals/cell; group 4, HER2/CEP17 ratio of less than 2.0 and 4.0 or more but less than 6.0 HER2 signals/cell; and group 5, HER2/CEP17 ratio less than 2.0 and less than 4.0 HER2 signals/cell. Group 1 was reported as HER2 FISH amplified/positive, whereas group 5 was the negative group. For groups 2 to 4, final HER2 results were based on concurrent review of the HER2 IHC slides with recounting of the FISH slides by another reviewer if HER2 IHC was 2+. Subsequently, tumors were designated as HER2 amplified/positive if the following criteria were fulfilled: group 2 and concurrent IHC 3+, group 3 and concurrent IHC 2+ or 3+, and group 4 and concurrent IHC 3+. Tumors were designated as HER2 nonamplified/negative (FISH assay) if the following criteria were met: group 2 and concurrent IHC 0 to 1+ or 2+, group 3 and concurrent IHC 0 to 1+, and group 4 and concurrent IHC 0 to 1+ or 2+.28
Statistical Analysis
Fisher exact test and Pearson χ 2 tests were performed to determine the association between clinicopathologic features and HER2 amplification, as well as to compare the HER2 concordance as per ASCO/CAP guidelines of 2013 vs 2018.
Results
Clinicopathologic Characteristics
A total of 78 cases of HGUCa were included in this study. There were 54 men and 24 women. Their ages ranged from 27 to 85 years, with a median age of 59 years. Pure HGUCa histology was observed in 35. The remaining tumors had the following histologies: HGUCa with MP pattern, n = 23; HGUCa with squamous features, n = 9; HGUCa with glandular differentiation, n = 4; HGUCa with small cell features, n = 2; HGUCa with plasmacytoid features, n = 1; HGUCa with nested pattern, n = 1; and small cell carcinoma, n = 3. There were 5 stage I tumors, 50 stage II tumors, 10 stage III tumors, and 13 stage IV tumors Table 1. There were 26 (33.3%) basal type, 39 (50%) luminal type, and 13 (16.7%) nonluminal and nonbasal or basoluminal type tumors.
| Characteristic . | Value . |
|---|---|
| Age, median (range), y | 59 (27-85) |
| Sex, No. (%) | |
| Male | 54 (69.2) |
| Female | 24 (30.8) |
| Stage, No. (%) | |
| I | 5 (6.4) |
| II | 50 (64.1) |
| III | 10 (12.8) |
| IV | 13 (16.7) |
| Histopathologic subtype, No. (%) | |
| HGUCa | 35 (44.87) |
| HGUCa with micropapillary features | 23 (29.48) |
| HGUCa with glandular features | 4 (5.12) |
| HGUCa with squamous features | 9 (11.5) |
| HGUCa with small cell features | 2 (2.56) |
| Nested variant of HGUCa | 1 (1.28) |
| Plasmacytoid variant of HGUCa | 1 (1.28) |
| Small cell carcinoma | 3 (3.84) |
| Molecular subtype, No. (%) | |
| Basal | 26 (33.3) |
| Luminal | 39 (50.0) |
| Nonluminal and nonbasal/basoluminal | 13 (16.7) |
| Characteristic . | Value . |
|---|---|
| Age, median (range), y | 59 (27-85) |
| Sex, No. (%) | |
| Male | 54 (69.2) |
| Female | 24 (30.8) |
| Stage, No. (%) | |
| I | 5 (6.4) |
| II | 50 (64.1) |
| III | 10 (12.8) |
| IV | 13 (16.7) |
| Histopathologic subtype, No. (%) | |
| HGUCa | 35 (44.87) |
| HGUCa with micropapillary features | 23 (29.48) |
| HGUCa with glandular features | 4 (5.12) |
| HGUCa with squamous features | 9 (11.5) |
| HGUCa with small cell features | 2 (2.56) |
| Nested variant of HGUCa | 1 (1.28) |
| Plasmacytoid variant of HGUCa | 1 (1.28) |
| Small cell carcinoma | 3 (3.84) |
| Molecular subtype, No. (%) | |
| Basal | 26 (33.3) |
| Luminal | 39 (50.0) |
| Nonluminal and nonbasal/basoluminal | 13 (16.7) |
HGUCa, high-grade urothelial carcinoma.
| Characteristic . | Value . |
|---|---|
| Age, median (range), y | 59 (27-85) |
| Sex, No. (%) | |
| Male | 54 (69.2) |
| Female | 24 (30.8) |
| Stage, No. (%) | |
| I | 5 (6.4) |
| II | 50 (64.1) |
| III | 10 (12.8) |
| IV | 13 (16.7) |
| Histopathologic subtype, No. (%) | |
| HGUCa | 35 (44.87) |
| HGUCa with micropapillary features | 23 (29.48) |
| HGUCa with glandular features | 4 (5.12) |
| HGUCa with squamous features | 9 (11.5) |
| HGUCa with small cell features | 2 (2.56) |
| Nested variant of HGUCa | 1 (1.28) |
| Plasmacytoid variant of HGUCa | 1 (1.28) |
| Small cell carcinoma | 3 (3.84) |
| Molecular subtype, No. (%) | |
| Basal | 26 (33.3) |
| Luminal | 39 (50.0) |
| Nonluminal and nonbasal/basoluminal | 13 (16.7) |
| Characteristic . | Value . |
|---|---|
| Age, median (range), y | 59 (27-85) |
| Sex, No. (%) | |
| Male | 54 (69.2) |
| Female | 24 (30.8) |
| Stage, No. (%) | |
| I | 5 (6.4) |
| II | 50 (64.1) |
| III | 10 (12.8) |
| IV | 13 (16.7) |
| Histopathologic subtype, No. (%) | |
| HGUCa | 35 (44.87) |
| HGUCa with micropapillary features | 23 (29.48) |
| HGUCa with glandular features | 4 (5.12) |
| HGUCa with squamous features | 9 (11.5) |
| HGUCa with small cell features | 2 (2.56) |
| Nested variant of HGUCa | 1 (1.28) |
| Plasmacytoid variant of HGUCa | 1 (1.28) |
| Small cell carcinoma | 3 (3.84) |
| Molecular subtype, No. (%) | |
| Basal | 26 (33.3) |
| Luminal | 39 (50.0) |
| Nonluminal and nonbasal/basoluminal | 13 (16.7) |
HGUCa, high-grade urothelial carcinoma.
Of the 78 tumors, using both 2013 and 2018 ASCO/CAP guidelines for HER2 IHC interpretation, 22 were positive (3+), 6 were equivocal (2+), and 50 were negative (0; 1+) Figure 1. HER2 amplification by FISH was observed in 22 (28.2%) of cases (ASCO/CAP, 2018), which was more frequent in MP and squamous histologies, compared with conventional HGUCa (20 vs 2; P < .00001). None of the small cell carcinoma cases were HER2 amplified Table 2. HER2 amplification did not show significant association with age, sex, molecular subtype, and tumor stage (Table 1). Twenty-six (33.3%), 39 (50%), and 13 (16.7%) tumors belonged to basal, luminal, and nonluminal and nonbasal/basoluminal categories, respectively. Of the basal tumors, 19 (24.3%) were HER2 amplified and 7 (9%) were HER2 nonamplified (ASCO/CAP, 2018). Twenty-six (33.3%) luminal and 11 (14.1%) nonluminal and nonbasal/basoluminal tumors were HER2 amplified, whereas 13 (16.7%) luminal and 2 (2.6%) nonluminal and nonbasal/basoluminal tumors were HER2-nonamplified (ASCO/CAP, 2018) (P = .45) (Table 2).
| Clinicopathologic Characteristic . | HER2 Nonamplified (ASCO/CAP, 2018) . | HER2 Amplified (ASCO/CAP, 2018) . | P Value . |
|---|---|---|---|
| Age, No. (%) | .46 | ||
| ≤50 y | 18 (23.1) | 9 (11.5) | |
| >50 y | 38 (48.7) | 13 (16.7) | |
| Sex, No. (%) | .22 | ||
| Male | 15 (19.2) | 9 (11.5) | |
| Female | 41 (15.6) | 13 (16.7) | |
| Stage, No. (%) | .37 | ||
| I | 4 (5.2) | 1 (1.3) | |
| II | 38 (48.7) | 12 (15.4) | |
| III | 5 (6.4) | 5 (6.4) | |
| IV | 9 (11.5) | 4 (5.1) | |
| Histopathologic subtype, No. | <.00001 | ||
| HGUCa | 34 | 1 | |
| HGUCa with micropapillary features | 8 | 15 | |
| HGUCa with glandular features | 4 | 0 | |
| HGUCa with squamous features | 4 | 5 | |
| HGUCa with small cell features | 2 | 0 | |
| Nested variant of HGUCa | 1 | 0 | |
| Plasmacytoid variant of HGUCa | 0 | 1 | |
| Small cell carcinoma | 3 | 0 | |
| Molecular subtypes, No. (%) | .45 | ||
| Basal | 7 (9.0) | 19 (24.3) | |
| Luminal | 13 (16.7) | 26 (33.3) | |
| Nonluminal and nonbasal/basoluminal | 2 (2.6) | 11 (14.1) |
| Clinicopathologic Characteristic . | HER2 Nonamplified (ASCO/CAP, 2018) . | HER2 Amplified (ASCO/CAP, 2018) . | P Value . |
|---|---|---|---|
| Age, No. (%) | .46 | ||
| ≤50 y | 18 (23.1) | 9 (11.5) | |
| >50 y | 38 (48.7) | 13 (16.7) | |
| Sex, No. (%) | .22 | ||
| Male | 15 (19.2) | 9 (11.5) | |
| Female | 41 (15.6) | 13 (16.7) | |
| Stage, No. (%) | .37 | ||
| I | 4 (5.2) | 1 (1.3) | |
| II | 38 (48.7) | 12 (15.4) | |
| III | 5 (6.4) | 5 (6.4) | |
| IV | 9 (11.5) | 4 (5.1) | |
| Histopathologic subtype, No. | <.00001 | ||
| HGUCa | 34 | 1 | |
| HGUCa with micropapillary features | 8 | 15 | |
| HGUCa with glandular features | 4 | 0 | |
| HGUCa with squamous features | 4 | 5 | |
| HGUCa with small cell features | 2 | 0 | |
| Nested variant of HGUCa | 1 | 0 | |
| Plasmacytoid variant of HGUCa | 0 | 1 | |
| Small cell carcinoma | 3 | 0 | |
| Molecular subtypes, No. (%) | .45 | ||
| Basal | 7 (9.0) | 19 (24.3) | |
| Luminal | 13 (16.7) | 26 (33.3) | |
| Nonluminal and nonbasal/basoluminal | 2 (2.6) | 11 (14.1) |
ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; HGUCa, high-grade urothelial carcinoma.
| Clinicopathologic Characteristic . | HER2 Nonamplified (ASCO/CAP, 2018) . | HER2 Amplified (ASCO/CAP, 2018) . | P Value . |
|---|---|---|---|
| Age, No. (%) | .46 | ||
| ≤50 y | 18 (23.1) | 9 (11.5) | |
| >50 y | 38 (48.7) | 13 (16.7) | |
| Sex, No. (%) | .22 | ||
| Male | 15 (19.2) | 9 (11.5) | |
| Female | 41 (15.6) | 13 (16.7) | |
| Stage, No. (%) | .37 | ||
| I | 4 (5.2) | 1 (1.3) | |
| II | 38 (48.7) | 12 (15.4) | |
| III | 5 (6.4) | 5 (6.4) | |
| IV | 9 (11.5) | 4 (5.1) | |
| Histopathologic subtype, No. | <.00001 | ||
| HGUCa | 34 | 1 | |
| HGUCa with micropapillary features | 8 | 15 | |
| HGUCa with glandular features | 4 | 0 | |
| HGUCa with squamous features | 4 | 5 | |
| HGUCa with small cell features | 2 | 0 | |
| Nested variant of HGUCa | 1 | 0 | |
| Plasmacytoid variant of HGUCa | 0 | 1 | |
| Small cell carcinoma | 3 | 0 | |
| Molecular subtypes, No. (%) | .45 | ||
| Basal | 7 (9.0) | 19 (24.3) | |
| Luminal | 13 (16.7) | 26 (33.3) | |
| Nonluminal and nonbasal/basoluminal | 2 (2.6) | 11 (14.1) |
| Clinicopathologic Characteristic . | HER2 Nonamplified (ASCO/CAP, 2018) . | HER2 Amplified (ASCO/CAP, 2018) . | P Value . |
|---|---|---|---|
| Age, No. (%) | .46 | ||
| ≤50 y | 18 (23.1) | 9 (11.5) | |
| >50 y | 38 (48.7) | 13 (16.7) | |
| Sex, No. (%) | .22 | ||
| Male | 15 (19.2) | 9 (11.5) | |
| Female | 41 (15.6) | 13 (16.7) | |
| Stage, No. (%) | .37 | ||
| I | 4 (5.2) | 1 (1.3) | |
| II | 38 (48.7) | 12 (15.4) | |
| III | 5 (6.4) | 5 (6.4) | |
| IV | 9 (11.5) | 4 (5.1) | |
| Histopathologic subtype, No. | <.00001 | ||
| HGUCa | 34 | 1 | |
| HGUCa with micropapillary features | 8 | 15 | |
| HGUCa with glandular features | 4 | 0 | |
| HGUCa with squamous features | 4 | 5 | |
| HGUCa with small cell features | 2 | 0 | |
| Nested variant of HGUCa | 1 | 0 | |
| Plasmacytoid variant of HGUCa | 0 | 1 | |
| Small cell carcinoma | 3 | 0 | |
| Molecular subtypes, No. (%) | .45 | ||
| Basal | 7 (9.0) | 19 (24.3) | |
| Luminal | 13 (16.7) | 26 (33.3) | |
| Nonluminal and nonbasal/basoluminal | 2 (2.6) | 11 (14.1) |
ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; HGUCa, high-grade urothelial carcinoma.
A, High-grade urothelial carcinoma (HGUCa) (H&E, ×10). B, HGUCa with 3+ HER2 staining (overexpression) (HER2/neu IHC, ×10). C, HGUCa with micropapillary features (H&E, ×10). D, HGUCa with micropapillary features and 3+ HER2 staining (overexpression) (HER2/neu IHC, ×10). E, HGUCa with 2+ HER2 staining (equivocal) (HER2/neu IHC, ×10). F, HGUCa with 2+ HER2 staining (equivocal) (HER2/neu IHC, ×20).
HER2 Result by FISH Using 2013 ASCO/CAP Criteria
Of the 78 tumors, 36 (46.2%) had HER2 amplification (HER2/CEP17 <2 with average HER2 copy number per cell ≥6, n = 3; HER2/CEP17 ≥2 and average HER2 copy number ≥4, n = 17; and HER2/CEP17 ratio ≥2 and average HER2 copy number <4, n = 16), 37 (47.4%) were negative for HER2 amplification (average HER2 copy number per cell <4 and HER2/CEP17 <2), and 5 (6.4%) tumors showed an equivocal result (average HER2 copy number per cell between ≥4 and 6 and HER2/CEP17 <2).
HER2 Result by FISH Using 2018 ASCO/CAP Criteria
When the above tumors were reevaluated using 2018 guidelines, 22 (28.2%) tumors were HER2 amplified and 56 (71.8%) were negative for HER2 amplification. The in situ hybridization assay data, when categorized into the five groups as mentioned in Table 1 per the ASCO/CAP 2018 guidelines, showed that 17 cases fell into group 1, 16 into group 2, 3 into group 3, 5 into group 4, and 37 into group 5 Table 3 and Figure 2. All the 22 FISH-amplified tumors (ASCO/CAP 2018) exhibited 3+ staining by IHC.
HER2 Fluorescence In Situ Hybridization and IHC Results per 2018 Guidelines in the Cohort
| Group . | HER2/CEP17 Ratio . | HER2 Signals/Cell . | No. of Cases . | HER2 IHC Result (No. of Cases) . | Consensus/Overall HER2 Result (No. of Cases) . | HER2 IHC Equivocal (2+) and Positive (3+) Regions With the Tumors (No. of Cases) . |
|---|---|---|---|---|---|---|
| 1 | ≥2 | ≥4 | 17 | 3+ (17) | Positive (17) | Urothelial and micropapillary areas (13) |
| Urothelial and squamous areas (2) | ||||||
| Urothelial and plasmacytoid areas (1) | ||||||
| Urothelial area (1) | ||||||
| 2 | ≥2 | <4 | 16 | 0 (3); 1+ (8); 2+ (4) | Negative (15) | For the 3+ case: Urothelial and micropapillary area |
| 3+ (1) | Positive (1) | For the 2+ cases: Urothelial and squamous areas (1); urothelial area (3) | ||||
| 3 | <2 | ≥6 | 2 | 3+ (2) | Positive (2) | Micropapillary area (1) |
| Squamous area (1) | ||||||
| 4 | <2 | <6 and ≥4 | 6 | 0 (1); 1+ (1); 2+ (2) | Negative (4) | For the 3+ cases: Squamous area (1); urothelial and squamous areas (1) |
| 3+ (2) | Positive (2) | For the 2+ cases: Urothelial areas (2) | ||||
| 5 | <2 | <4 | 37 | 1+ (3); 0 (34) | Negative (37) | Not applicable |
| Group . | HER2/CEP17 Ratio . | HER2 Signals/Cell . | No. of Cases . | HER2 IHC Result (No. of Cases) . | Consensus/Overall HER2 Result (No. of Cases) . | HER2 IHC Equivocal (2+) and Positive (3+) Regions With the Tumors (No. of Cases) . |
|---|---|---|---|---|---|---|
| 1 | ≥2 | ≥4 | 17 | 3+ (17) | Positive (17) | Urothelial and micropapillary areas (13) |
| Urothelial and squamous areas (2) | ||||||
| Urothelial and plasmacytoid areas (1) | ||||||
| Urothelial area (1) | ||||||
| 2 | ≥2 | <4 | 16 | 0 (3); 1+ (8); 2+ (4) | Negative (15) | For the 3+ case: Urothelial and micropapillary area |
| 3+ (1) | Positive (1) | For the 2+ cases: Urothelial and squamous areas (1); urothelial area (3) | ||||
| 3 | <2 | ≥6 | 2 | 3+ (2) | Positive (2) | Micropapillary area (1) |
| Squamous area (1) | ||||||
| 4 | <2 | <6 and ≥4 | 6 | 0 (1); 1+ (1); 2+ (2) | Negative (4) | For the 3+ cases: Squamous area (1); urothelial and squamous areas (1) |
| 3+ (2) | Positive (2) | For the 2+ cases: Urothelial areas (2) | ||||
| 5 | <2 | <4 | 37 | 1+ (3); 0 (34) | Negative (37) | Not applicable |
IHC, immunohistochemistry.
HER2 Fluorescence In Situ Hybridization and IHC Results per 2018 Guidelines in the Cohort
| Group . | HER2/CEP17 Ratio . | HER2 Signals/Cell . | No. of Cases . | HER2 IHC Result (No. of Cases) . | Consensus/Overall HER2 Result (No. of Cases) . | HER2 IHC Equivocal (2+) and Positive (3+) Regions With the Tumors (No. of Cases) . |
|---|---|---|---|---|---|---|
| 1 | ≥2 | ≥4 | 17 | 3+ (17) | Positive (17) | Urothelial and micropapillary areas (13) |
| Urothelial and squamous areas (2) | ||||||
| Urothelial and plasmacytoid areas (1) | ||||||
| Urothelial area (1) | ||||||
| 2 | ≥2 | <4 | 16 | 0 (3); 1+ (8); 2+ (4) | Negative (15) | For the 3+ case: Urothelial and micropapillary area |
| 3+ (1) | Positive (1) | For the 2+ cases: Urothelial and squamous areas (1); urothelial area (3) | ||||
| 3 | <2 | ≥6 | 2 | 3+ (2) | Positive (2) | Micropapillary area (1) |
| Squamous area (1) | ||||||
| 4 | <2 | <6 and ≥4 | 6 | 0 (1); 1+ (1); 2+ (2) | Negative (4) | For the 3+ cases: Squamous area (1); urothelial and squamous areas (1) |
| 3+ (2) | Positive (2) | For the 2+ cases: Urothelial areas (2) | ||||
| 5 | <2 | <4 | 37 | 1+ (3); 0 (34) | Negative (37) | Not applicable |
| Group . | HER2/CEP17 Ratio . | HER2 Signals/Cell . | No. of Cases . | HER2 IHC Result (No. of Cases) . | Consensus/Overall HER2 Result (No. of Cases) . | HER2 IHC Equivocal (2+) and Positive (3+) Regions With the Tumors (No. of Cases) . |
|---|---|---|---|---|---|---|
| 1 | ≥2 | ≥4 | 17 | 3+ (17) | Positive (17) | Urothelial and micropapillary areas (13) |
| Urothelial and squamous areas (2) | ||||||
| Urothelial and plasmacytoid areas (1) | ||||||
| Urothelial area (1) | ||||||
| 2 | ≥2 | <4 | 16 | 0 (3); 1+ (8); 2+ (4) | Negative (15) | For the 3+ case: Urothelial and micropapillary area |
| 3+ (1) | Positive (1) | For the 2+ cases: Urothelial and squamous areas (1); urothelial area (3) | ||||
| 3 | <2 | ≥6 | 2 | 3+ (2) | Positive (2) | Micropapillary area (1) |
| Squamous area (1) | ||||||
| 4 | <2 | <6 and ≥4 | 6 | 0 (1); 1+ (1); 2+ (2) | Negative (4) | For the 3+ cases: Squamous area (1); urothelial and squamous areas (1) |
| 3+ (2) | Positive (2) | For the 2+ cases: Urothelial areas (2) | ||||
| 5 | <2 | <4 | 37 | 1+ (3); 0 (34) | Negative (37) | Not applicable |
IHC, immunohistochemistry.
![HER2 fluorescence in situ hybridization (FISH) in high-grade urothelial carcinoma: A, Group 1 = HER2/CEP17 ratio of 2.0 or more and 4.0 or more HER2 signals/cell. B, Group 2 = HER2/CEP17 ratio of 2.0 or more and less than 4.0 HER2 signals/cell. C, Group 3 = HER2/CEP17 ratio of less than 2.0 and 6.0 or more HER2 signals/cell. D, Group 4 = HER2/CEP17 ratio of less than 2.0 and 4.0 or more and less than 6.0 HER2 signals/cell. E, Group 5 = HER2/CEP17 ratio of less than 2.0 and less than 4.0 HER2 signals/cell (orange signal [HER]; green signal [CEP17]) (FISH, ×60).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ajcp/156/6/10.1093_ajcp_aqab083/2/m_aqab083_fig2.jpeg?Expires=1750309381&Signature=0OMZtVXKvPCCHBe2c85rljP2yuEoxBPLjEJAiJ2h0cnpjmJhWEgaeU-k0vTrcAK1abdelMexWsBTgqKgr-sIXzfkOAF1tPCWpbEDCh2Fzk0RIAhs4UKfF5fXKbWy9Yp2-YIREZuIgsyLMQTp2PD3Rma5X9t8q-~VQ1p1k-3H663G~oO-54nDqV3OsQ71OF4qskCXXz6AVgl2b0qede7zhwhdFaTINj9GQ-QpByecE5zdNUCZTqwPuALs-c2tAMtKLeCxwaXVBcILsRK0dTxGDuZRXocTWmn~b1wUMCPXdD5wI8aTsKgd1q628m5yvWW1pvqviiJs29BMFyfhfJjeyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
HER2 fluorescence in situ hybridization (FISH) in high-grade urothelial carcinoma: A, Group 1 = HER2/CEP17 ratio of 2.0 or more and 4.0 or more HER2 signals/cell. B, Group 2 = HER2/CEP17 ratio of 2.0 or more and less than 4.0 HER2 signals/cell. C, Group 3 = HER2/CEP17 ratio of less than 2.0 and 6.0 or more HER2 signals/cell. D, Group 4 = HER2/CEP17 ratio of less than 2.0 and 4.0 or more and less than 6.0 HER2 signals/cell. E, Group 5 = HER2/CEP17 ratio of less than 2.0 and less than 4.0 HER2 signals/cell (orange signal [HER]; green signal [CEP17]) (FISH, ×60).
Comparison Between the HER2 Results Using 2013 and 2018 ASCO/CAP Guidelines
Thirty-seven FISH nonamplified tumors using 2013 criteria remained nonamplified (group 5, 2018). Seventeen HER2-amplified tumors per 2013 remained amplified (group 1, 2018). These tumors were IHC 3+. Three HER2-amplified tumors per 2013 remained amplified (group 3, 2018) as the IHC was 3+ in these tumors. One HER2-amplified tumor per 2013 remained amplified (group 2, 2018) as the IHC was 3+. The remaining 15 HER2-amplified tumors per 2013 guidelines became negative as they fell into group 2 (2018); however, these were either negative (0, n = 3 or 1+, n = 1) or equivocal (2+, n = 4) on IHC evaluation. Five FISH-equivocal (group 4, 2018) tumors per 2013 criteria were further categorized into HER2 amplified (n = 1, as IHC was 3+) and HER2 nonamplified (n = 4, as IHC was either negative [0, n = 1; 1+, n = 1] or equivocal [2+, n = 2]) Table 4 and Figure 3.
Comparative HER2 FISH Distribution Based on ASCO/CAP 2013 and ASCO/CAP 2018 Guidelines
| HER2 FISH . | Result per 2013 ASCO/CAP Guidelines, No. (%) . | Result per 2018 ASCO/CAP Guidelines, No. (%) . |
|---|---|---|
| Amplified | 36 (46.1) | 22 (28.2)a |
| Equivocal | 5 (6.4) | Not applicable |
| Nonamplified | 37 (47.5) | 56 (71.8)b |
| HER2 FISH . | Result per 2013 ASCO/CAP Guidelines, No. (%) . | Result per 2018 ASCO/CAP Guidelines, No. (%) . |
|---|---|---|
| Amplified | 36 (46.1) | 22 (28.2)a |
| Equivocal | 5 (6.4) | Not applicable |
| Nonamplified | 37 (47.5) | 56 (71.8)b |
ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; FISH, fluorescence in situ hybridization.
aIn total, 17.9% became nonamplified.
bIn total, 24.3% became amplified.
Comparative HER2 FISH Distribution Based on ASCO/CAP 2013 and ASCO/CAP 2018 Guidelines
| HER2 FISH . | Result per 2013 ASCO/CAP Guidelines, No. (%) . | Result per 2018 ASCO/CAP Guidelines, No. (%) . |
|---|---|---|
| Amplified | 36 (46.1) | 22 (28.2)a |
| Equivocal | 5 (6.4) | Not applicable |
| Nonamplified | 37 (47.5) | 56 (71.8)b |
| HER2 FISH . | Result per 2013 ASCO/CAP Guidelines, No. (%) . | Result per 2018 ASCO/CAP Guidelines, No. (%) . |
|---|---|---|
| Amplified | 36 (46.1) | 22 (28.2)a |
| Equivocal | 5 (6.4) | Not applicable |
| Nonamplified | 37 (47.5) | 56 (71.8)b |
ASCO, American Society of Clinical Oncology; CAP, College of American Pathologists; FISH, fluorescence in situ hybridization.
aIn total, 17.9% became nonamplified.
bIn total, 24.3% became amplified.

A, HER2 fluorescence in situ hybridization (FISH) amplification status per American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP), 2013 (amplified, n = 36; nonamplified, n = 37; equivocal, n = 5). B, HER2 FISH amplification status per ASCO/CAP, 2018 (amplified, n = 22; nonamplified, n = 56). C, Comparison between HER2 FISH results per 2013 and 2018 ASCO/CAP guidelines (of the 5 HER2-equivocal tumors per ASCO/CAP 2013, 1 was amplified and 4 were nonamplified per ASCO/CAP, 2018; all HER2 nonamplified tumors per ASCO/CAP, 2013 remained nonamplified per ASCO/CAP, 2018; of the 36 HER2-amplified tumors per ASCO/CAP, 2013, 15 were amplified and 21 were nonamplified per ASCO/CAP, 2018.
Summary of Results
In total, 20 (25.6%) tumors had discordant HER2 FISH results per 2018 ASCO/CAP guidelines compared with 2013 ASCO/CAP guidelines. In group 2, 15 of 16 positive tumors per 2013 ASCO/CAP guidelines were negative according to 2018 ASCO/CAP guidelines. Group 3 tumors remained positive per both guidelines, whereas of the five equivocal tumors from group 4, four were recategorized as HER2 negative and one tumor as HER2 positive per 2018 guidelines.
In summary, 36 tumors were HER2 amplified per 2013 ASCO/CAP guidelines, of which 21 had 3+ staining, whereas 4 had 2+, 8 had 1+, and 3 had 0 HER2 IHC scores. When 2018 ASCO/CAP guidelines were applied, 22 tumors with 3+ IHC score were HER2 amplified, and none of the HER2-amplified tumors had a score below 3+ on IHC. None of the tumors with a 3+ HER2 IHC score was negative by FISH using the 2018 ASCO/CAP guidelines. One tumor with a 3+ HER2 IHC score and a HER2-equivocal status by 2013 ASCO/CAP FISH criteria turned out to be HER2 amplified using the 2018 ASCO/CAP guidelines.
Discussion
The frequency of HER2 amplification in bladder cancer is quite variable across multiple studies, ranging up to 80% based on various defined criteria for HER2 positivity.3,7-9,11-14 Traditionally, true-positive/amplified cases have a 3+ score, whereas true-negative/nonamplified cases have a 0 or 1+ score, and equivocal cases have a 2+ score. In the previously published clinical trials on UCa, cases with an IHC score of 1+ and 2+ were included, and FISH was not performed in all tumors and hence unlikely to benefit from anti-HER2 therapy.15,18,25HER2 gene alteration occurs in UCa by amplification and mutations.8-10 Although HER2 interpretation and reporting in breast cancers have evolved from 2007 to 2013 to 2018 to segregate the true-positive/amplified tumors from the negative ones, there is no such guideline for HER2 reporting in bladder cancers. This also makes it difficult to interpret the results of different clinical trials. Therefore, a properly designed algorithm and guideline is needed for HER2 reporting in bladder cancer.
The 2018 ASCO/CAP guidelines for HER2 reporting in breast cancer have homogenized the HER2 reporting with binary adjudication of results into positive or negative. Overall, per 2018 guidelines, 4.6% to 16% of HER2 FISH results in invasive breast carcinoma were reclassified, and this discordance was driven by an increase in HER2 FISH negativity (nonamplified) by 4% to 15.5%. At the same time, the reported positivity rate declined by 0.4% to 1.1%.29-31 We also had similar observations in HGUCa. Based on our results, it was observed that 20 (26%) tumors had discordant HER2 FISH results per the 2018 ASCO/CAP guidelines compared with the 2013 guidelines. There was an 18% decrement in the cases that were amplified and a 24% increment in cases that were nonamplified compared with the 2013 ASCO/CAP guidelines. The biggest alteration occurred in group 2 (HER2/CEP17 ratio ≥2 and HER2 signals/cell <4). Of patients previously reported as HER2 FISH amplified, 15 of 16 were reclassified as HER2 nonamplified. Since there has been no prior study on HER2 reporting using the 2018 guidelines in bladder carcinoma, the clinical and therapeutic implications of our observations need to be assessed, keeping in view the HER2 interpretation in breast carcinoma. Experts have expressed apprehensions at adjudicating HER2 results as amplified or nonamplified solely on the HER2/CEP17 ratio of 2 or more without taking into consideration the copy number of HER2 per cell, as was done in the initial trials of trastuzumab in breast cancer.28 This could result in overestimation in HER2 amplification with CEP17 loss and underestimation with CEP17 gain. In previous trastuzumab trials, group 2 patients comprised 0.4% to 3.7% of all cases, and on IHC testing, none of the patients were IHC 3+. Based on retrospective analysis, these patients were unlikely to derive any benefit from trastuzumab, suggesting they are true negative for HER2 amplification.32 Whether this is validated prospectively needs to be assessed in the future clinical trials. All five tumors in our cohort of HGUCa, reported as HER2 FISH equivocal (group 4) per 2013 guidelines, were adjudicated as either HER2 nonamplified (four tumors) or amplified (one tumor) per the new guideline (ASCO/CAP, 2018). The equivocal subset accounts for roughly 5% (range, 1%-16%) of tumors of invasive breast carcinoma.28,32-34 These groups of patients with breast carcinoma were by default ineligible for inclusion in anti-HER2 trials, and hence the benefit of such therapy is dubious. However, based on the clinical trial data, reflex IHC tests have shown 89% HER2 IHC negative (0, 1+), 10% equivocal (2+), and only 0.9% as HER2 IHC 3+.34 Another study on breast carcinoma showed that all these FISH-equivocal tumors were HER2 negative by IHC.35 Thus, most patients with HER2-equivocal results are true negative for HER2 amplification and hence unlikely to benefit from anti-HER2 therapy.
HER2 amplification was more frequent in MP and squamous histologies compared with conventional HGUCa (20 vs 2; P < .00001), which is in concordance with the previous observation by Moktefi et al.15
To our knowledge, there have been no prior studies on the impact of the 2018 ASCO/CAP guidelines on HER2 reporting in bladder carcinoma. Based on our results, all patients with HER2 amplification could be reclassified as HER2 positive (amplified) or negative (nonamplified), thus obviating the need to interpret results as equivocal. There was, however, an 18% decrement in HER2-amplified tumors per the 2018 guidelines. In breast cancer, this was in the range of 0.4% to 1.1%. This discrepancy among tumor types is unexplained and could be due to a greater degree of intratumoral heterogeneity and a higher incidence of CEP17 gains in bladder carcinoma.9 In the study by Moktefi et al,15 intratumoral heterogeneity for HER2 IHC was observed in 7% of primary tumors and 6% of lymph node metastases; 24% of positive HER2 FISH cases demonstrated intratumoral heterogeneity.
The principal focus and novelty of our study was to validate the 2018 ASCO/CAP HER2 testing and reporting guideline in HGUCa using both IHC and FISH platforms and compare the results with the 2013 ASCO/CAP guidelines. We also ascertained the utility of the 2018 guideline to correctly identify HER2-amplified bladder cancers from the nonamplified ones and the way to address the so-called HER2 FISH equivocal tumors per the 2013 guidelines. This would aid in stratifying the patients who would benefit from anti-HER2 therapy in HGUCa. We did not analyze the survival and follow-up information in our cohort as this was not the goal of our study. Based on our results and taking ASCO/CAP 2018 guidelines for HER2 reporting in invasive breast carcinoma as a model, we propose an algorithm that is illustrated in Figure 4.
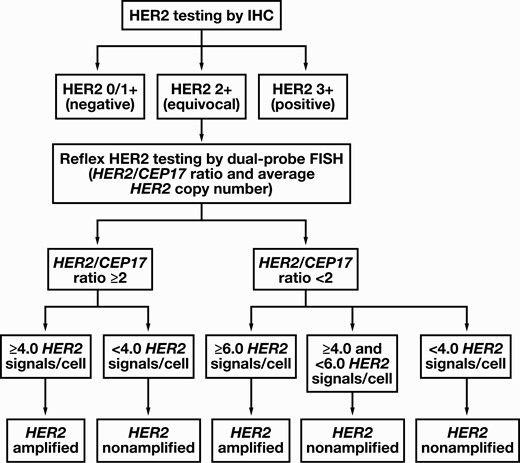
Algorithm for human epidermal growth factor receptor 2 (HER2) testing in high-grade urothelial carcinoma. First, immunohistochemistry (IHC) testing for HER2 should be performed. A fluorescence in situ hybridization (FISH) test is not required if the HER2 IHC test results are either negative (0 or 1+ score) or positive (3+ score), while a reflex FISH testing is recommended for HER2 IHC-equivocal (2+ score) tumors. The tumors with a 3+ score are considered HER2 amplified, and a FISH test is not mandatory. The FISH results are further divided based on HER2/CEP17 ratio into two broad categories as HER2/CEP17 ratio of 2 or more and HER2/CEP17 ratio of less than 2. Subsequently, depending on the average HER2 copy number, the tumors were further subtyped into five categories as follows: group 1, HER2/CEP17 ratio of 2.0 or more and 4.0 or more HER2 signals/cell; group 2, HER2/CEP17 ratio of 2.0 or more and less than 4.0 HER2 signals/cell; group 3, HER2/CEP17 ratio of less than 2.0 and 6.0 or more HER2 signals/cell; group 4, HER2/CEP17 ratio of less than 2.0 and 4.0 or more but less than 6.0 HER2 signals/cell; and group 5, HER2/CEP17 ratio of less than 2.0 and less than 4.0 HER2 signals/cell. A tumor with a 2+ score on HER2 IHC and either a group 1 or group 3 FISH pattern is considered HER2 amplified, whereas a tumor with a 2+ score on HER2 IHC and a group 2, 4, or 5 FISH pattern is considered HER2 nonamplified.
In conclusion, the standardized recategorization of FISH results into HER2 positive (amplified) and negative (nonamplified) per the 2018 guidelines should be used and validated in future clinical trials for anti-HER2 therapy in HGUCas of the urinary bladder.
Currently, there is a lack of literature on appropriate validation and standardized implementation of HER2 reporting in the HGUCa of the urinary bladder. Also, there are no data comparing HER2 reporting per 2013 with the 2018 ASCO/CAP guidelines in HGUCa.
Implementing 2018 guidelines, HER2 amplification decreased from 36 (46.1%, 2013 ASCO/CAP) to 22 (28.2%, 2018 ASCO/CAP) cases of HGUCa.
The group with a HER2/CEP17 ratio of 2 or more and average HER2 copy number of less than 4 (group 2) was predominantly negative by IHC and hence HER2 nonamplified, which may not respond to HER2-targeted therapy.
This study was presented as a poster at the USCAP Annual Meeting; March 18, 2019; National Harbor, MD, USA.
References
Author notes
First authors.






