-
PDF
- Split View
-
Views
-
Cite
Cite
Mary Kathryn Bohn, Alexandra Hall, Siobhan Wilson, Tina Henderson, Khosrow Adeli, Pediatric Reference Intervals for Critical Point-of-Care Whole Blood Assays in the CALIPER Cohort of Healthy Children and Adolescents, American Journal of Clinical Pathology, Volume 156, Issue 6, December 2021, Pages 1030–1037, https://doi.org/10.1093/ajcp/aqab064
Close - Share Icon Share
Abstract
Point-of-care testing (POCT) is being increasingly adopted to support clinical care. Data for critical care parameters in healthy children on POCT instruments are lacking. We established comprehensive reference standards for several whole blood parameters on the Radiometer ABL90 FLEX PLUS blood gas analyzer in the Canadian Laboratory Initiative on Paediatric Reference Intervals (CALIPER) cohort.
Approximately 300 healthy children and adolescents (age range, birth to <19 years; sex, boys and girls) were recruited with informed consent. Venous whole blood was collected (using heparinized syringes) and rapidly analyzed at the point of collection for pH, Pco2, Po2, carboxyhemoglobin, methemoglobin, lactate, and electrolytes on the ABL90 FLEX PLUS instrument. Reference intervals were established according to Clinical and Laboratory Standards Institute guidelines.
Of the parameters assessed, 6 required age partitioning; none required sex partitioning. Reference value distributions were consistent across the pediatric age range, demonstrating higher variation in the early neonatal period.
This study established reference standards for 10 critical care analytes in the CALIPER cohort for the first time. These data contribute to our understanding of normative pediatric values for venous electrolytes, metabolites, and blood gases on a modern POCT instrument, facilitating test interpretation in clinical settings that use these assays.
Reference intervals were established for 10 critical care parameters (venous whole blood) in the CALIPER cohort of healthy children on the Radiometer ABL90 FLEX PLUS instrument.
Of the 10 parameters assessed, 6 required age partitioning and none required sex partitioning, with consistent reference value distributions across the pediatric age range.
Study findings will help facilitate accurate interpretation of critical care test results on POCT platforms with emerging clinical prominence and utility.
Point-of-care testing (POCT) systems allow for the measurement of several critical care parameters in near-patient settings, ideally reducing laboratory turnaround time without compromising accuracy and thus improving timely clinical decision-making.1 As clinical institutions begin to adopt a more patient-centered approach to medicine, POCT is rapidly becoming an essential laboratory service, particularly for critical care units and emergency departments. Common analytes currently measured by POCT systems include electrolytes (eg, sodium, potassium, chloride, ionized calcium), metabolites (eg, glucose and lactate), and blood gases, with an increasing trend toward incorporating other chemistry parameters (eg, total bilirubin and creatinine).2 Accurate interpretation of laboratory test results for these parameters on POCT systems requires both reliable results and accurate reference standards.3,4 Reference standards, also known as reference intervals (RIs), are defined as the central 95% of laboratory test results observed in a healthy reference population and are used to flag abnormal results and assist in clinical decision-making.4 Recent studies support good analytical comparability between POCT systems and core laboratory analyzers for many parameters,5-7 but available data regarding health-associated reference standards for pediatric and adult test interpretation on these systems are minimal. Indeed, most available data are derived from textbooks, outdated analytical platforms, or expert consensus. The availability of robust, evidence-based reference standards for critical care parameters on POCT systems is of high clinical importance given their increasing role in clinical monitoring and treatment decisions.
The Canadian Laboratory Initiative on Paediatric Reference Intervals (CALIPER) study aims to address remaining gaps in pediatric RIs for clinically important biomarkers of health and disease.8 To date, CALIPER has recruited more than 12,000 healthy individuals from birth to 18 years of age in the community and established reference standards for more than 180 biomarkers on core laboratory analyzers from multiple manufacturers (ie, Abbott ARCHITECT9-12; Beckman Coulter UniCel DxI and DxC AU13-16; Ortho Clinical Diagnostics VITROS16-18; Roche Diagnostics cobas and modular16,19; Siemens Healthineers Dimension Vista, ADVIA, and Dimension EXL16,20). These comprehensive reference value data sets have greatly contributed to our knowledge of pediatric health-associated benchmarks for common laboratory tests and resulted in a novel online database that hundreds of institutions globally have accessed (www.caliperdatabase.org).8,21 Despite the richness of this database and others worldwide, evidence gaps remain for specialized parameters and analytical platforms, including critical care analytes on POCT systems. The establishment of reference standards for critical care analytes on POCT systems is complicated by challenges associated with recruiting a sufficiently large healthy pediatric population. In addition, unlike most biochemical and immunochemical parameters, where frozen serum aliquots can be used, POCT requires that fresh whole blood be analyzed within 30 minutes at the point of collection.
To address this gap, this study established pediatric reference standards for 10 critical care parameters (venous) on the widely used Radiometer ABL90 FLEX PLUS blood gas analyzer in the CALIPER cohort of healthy children and adolescents for the first time. These data greatly contribute to our knowledge of health-associated pediatric reference values for critical care parameters on the ABL90 FLEX PLUS instrument and can be expected to assist test interpretation in laboratories that use this platform.
Materials and Methods
Participant and Sample Acquisition
This study was approved by the Research Ethics Board at the Hospital for Sick Children, Toronto, Canada. Healthy children aged 5 to younger than 19 years were recruited as part of the CALIPER project to participate through community initiatives (eg, schools, community centers, daycares) in the Greater Toronto Area. Participation involved written informed consent, completion of a short health questionnaire, anthropometric measurements, and the donation of a small blood sample, as previously described.8 Whole blood (venous) was collected in safePICO syringes (Radiometer). These syringes include a self-sealing, vented safeTIPCAP that improves the removal of air bubbles before sample analysis. The syringe is also equipped with a metal mixing ball to ensure homogenous sample mixing. Outpatient samples from children aged birth to younger than 5 years from metabolically stable clinics were also used for analysis. All samples were mixed using the mechanical mixing apparatus present on the ABL90 FLEX PLUS instrument before analysis.
Sample Analysis
Whole blood samples (venous) were analyzed rapidly for 10 critical care parameters (ie, pH, Po2, Pco2, methemoglobin, carboxyhemoglobin, potassium, sodium, ionized calcium, chloride, lactate) within 30 minutes of collection on the ABL90 FLEX PLUS analyzer. Analytical methods were controlled according to the manufacturer’s instructions by preventive maintenance, function checks, calibration, and quality control (Supplemental Table 1; all supplemental material can be found at American Journal of Clinical Pathology online).
Statistical Analysis
Data were analyzed in accordance with Clinical and Laboratory Standards Institute (CLSI) EP28-A3 guidelines using Microsoft Excel and R, version 3.5.1 (R Foundation for Statistical Computing).4 Briefly, data were assessed visually by using scatterplots of analyte concentration plotted by age and color-coded by sex. Age- and sex-specific partitions were determined via the statistical method of Harris and Boyd.22 Outliers were then removed from each established partition using the Tukey method for Gaussian distributions and adjusted Tukey method for non-Gaussian distributions. Normality of data distribution was assessed using quantile-quantile plots and the Shapiro-Wilks test. We used the nonparametric rank method to calculate reference intervals for partitions that had a sample size greater than or equal to 120 participants.4 For partitions that had a sample size between 40 and 119 participants, we used the robust method of Horn and Pesce.23 We also calculated 90% confidence limits for both the upper and lower reference limits.
Results
Reference intervals were established for 10 critical care parameters, as outlined in Table 1. Of the 10 parameters studied, 6 required age partitioning (Po2, Pco2, carboxyhemoglobin, methemoglobin, chloride, and sodium). No statistical differences were observed between sexes Figure 1 and Figure 2.
Reference Intervals for 10 Critical POCT Parameters in the CALIPER Cohort of Healthy Children and Adolescents on the Radiometer ABL90 FLEX PLUS Blood Gas Analyzer
| Analyte, Units . | Age Partition . | Sample Size . | Lower Limit (90% CI) . | Upper Limit (90% CI) . |
|---|---|---|---|---|
| pH | Birth to <19 y | 279 | 7.31 (7.31-7.32) | 7.41 (7.41-7.42) |
| Pco2, mm Hg | Birth to <5 y | 42 | 40 (39-41) | 51 (50-53) |
| 5 to <19 y | 181 | 41 (39-42) | 61 (58-63) | |
| Po2, mm Hg | Birth to <5 y | 42 | 25 (23-28) | 49 (46-51) |
| 5 to <19 y | 180 | 15 (13-17) | 52 (46-59) | |
| Carboxyhemoglobin, % | Birth to <5 y | 95 | 0.4 (0.32-0.48) | 1.57 (1.49-1.65) |
| 5 to <19 y | 180 | 0.5 (0.4-0.5) | 0.9 (0.9-1.0) | |
| Methemoglobin, % | Birth to <5 y | 90 | 0.48 (0.44-0.54) | 1.45 (1.36-1.53) |
| 5 to <19 y | 184 | 0.6 (0.6-0.7) | 1.1 (1.0-1.2) | |
| Potassium, mmol/L | Birth to <19 y | 275 | 3.5 (3.4-3.5) | 4.7 (4.6-4.7) |
| Chloride, mmol/L | Birth to <1 y | 40 | 96 (94-97) | 108 (107-109) |
| 1 to <5 y | 56 | 101 (100-102) | 111 (110-112) | |
| 5 to <19 y | 184 | 101 (101-102) | 107 (107-108) | |
| Sodium, mmol/L | Birth to <5 y | 81 | 135 (134-136) | 143 (142-144) |
| 5 to <19 y | 150 | 138 (137-139) | 143 (143-143) | |
| Ionized calcium, mmol/L | Birth to <19 y | 268 | 1.19 (1.19-1.19) | 1.33 (1.31-1.35) |
| Lactate, mmol/L | Birth to <19 y | 271 | 0.6 (0.6-0.7) | 2.1 (1.8-2.3) |
| Analyte, Units . | Age Partition . | Sample Size . | Lower Limit (90% CI) . | Upper Limit (90% CI) . |
|---|---|---|---|---|
| pH | Birth to <19 y | 279 | 7.31 (7.31-7.32) | 7.41 (7.41-7.42) |
| Pco2, mm Hg | Birth to <5 y | 42 | 40 (39-41) | 51 (50-53) |
| 5 to <19 y | 181 | 41 (39-42) | 61 (58-63) | |
| Po2, mm Hg | Birth to <5 y | 42 | 25 (23-28) | 49 (46-51) |
| 5 to <19 y | 180 | 15 (13-17) | 52 (46-59) | |
| Carboxyhemoglobin, % | Birth to <5 y | 95 | 0.4 (0.32-0.48) | 1.57 (1.49-1.65) |
| 5 to <19 y | 180 | 0.5 (0.4-0.5) | 0.9 (0.9-1.0) | |
| Methemoglobin, % | Birth to <5 y | 90 | 0.48 (0.44-0.54) | 1.45 (1.36-1.53) |
| 5 to <19 y | 184 | 0.6 (0.6-0.7) | 1.1 (1.0-1.2) | |
| Potassium, mmol/L | Birth to <19 y | 275 | 3.5 (3.4-3.5) | 4.7 (4.6-4.7) |
| Chloride, mmol/L | Birth to <1 y | 40 | 96 (94-97) | 108 (107-109) |
| 1 to <5 y | 56 | 101 (100-102) | 111 (110-112) | |
| 5 to <19 y | 184 | 101 (101-102) | 107 (107-108) | |
| Sodium, mmol/L | Birth to <5 y | 81 | 135 (134-136) | 143 (142-144) |
| 5 to <19 y | 150 | 138 (137-139) | 143 (143-143) | |
| Ionized calcium, mmol/L | Birth to <19 y | 268 | 1.19 (1.19-1.19) | 1.33 (1.31-1.35) |
| Lactate, mmol/L | Birth to <19 y | 271 | 0.6 (0.6-0.7) | 2.1 (1.8-2.3) |
CALIPER, Canadian Laboratory Initiative on Paediatric Reference Intervals; CI, confidence interval; POCT, point-of-contact testing.
Reference Intervals for 10 Critical POCT Parameters in the CALIPER Cohort of Healthy Children and Adolescents on the Radiometer ABL90 FLEX PLUS Blood Gas Analyzer
| Analyte, Units . | Age Partition . | Sample Size . | Lower Limit (90% CI) . | Upper Limit (90% CI) . |
|---|---|---|---|---|
| pH | Birth to <19 y | 279 | 7.31 (7.31-7.32) | 7.41 (7.41-7.42) |
| Pco2, mm Hg | Birth to <5 y | 42 | 40 (39-41) | 51 (50-53) |
| 5 to <19 y | 181 | 41 (39-42) | 61 (58-63) | |
| Po2, mm Hg | Birth to <5 y | 42 | 25 (23-28) | 49 (46-51) |
| 5 to <19 y | 180 | 15 (13-17) | 52 (46-59) | |
| Carboxyhemoglobin, % | Birth to <5 y | 95 | 0.4 (0.32-0.48) | 1.57 (1.49-1.65) |
| 5 to <19 y | 180 | 0.5 (0.4-0.5) | 0.9 (0.9-1.0) | |
| Methemoglobin, % | Birth to <5 y | 90 | 0.48 (0.44-0.54) | 1.45 (1.36-1.53) |
| 5 to <19 y | 184 | 0.6 (0.6-0.7) | 1.1 (1.0-1.2) | |
| Potassium, mmol/L | Birth to <19 y | 275 | 3.5 (3.4-3.5) | 4.7 (4.6-4.7) |
| Chloride, mmol/L | Birth to <1 y | 40 | 96 (94-97) | 108 (107-109) |
| 1 to <5 y | 56 | 101 (100-102) | 111 (110-112) | |
| 5 to <19 y | 184 | 101 (101-102) | 107 (107-108) | |
| Sodium, mmol/L | Birth to <5 y | 81 | 135 (134-136) | 143 (142-144) |
| 5 to <19 y | 150 | 138 (137-139) | 143 (143-143) | |
| Ionized calcium, mmol/L | Birth to <19 y | 268 | 1.19 (1.19-1.19) | 1.33 (1.31-1.35) |
| Lactate, mmol/L | Birth to <19 y | 271 | 0.6 (0.6-0.7) | 2.1 (1.8-2.3) |
| Analyte, Units . | Age Partition . | Sample Size . | Lower Limit (90% CI) . | Upper Limit (90% CI) . |
|---|---|---|---|---|
| pH | Birth to <19 y | 279 | 7.31 (7.31-7.32) | 7.41 (7.41-7.42) |
| Pco2, mm Hg | Birth to <5 y | 42 | 40 (39-41) | 51 (50-53) |
| 5 to <19 y | 181 | 41 (39-42) | 61 (58-63) | |
| Po2, mm Hg | Birth to <5 y | 42 | 25 (23-28) | 49 (46-51) |
| 5 to <19 y | 180 | 15 (13-17) | 52 (46-59) | |
| Carboxyhemoglobin, % | Birth to <5 y | 95 | 0.4 (0.32-0.48) | 1.57 (1.49-1.65) |
| 5 to <19 y | 180 | 0.5 (0.4-0.5) | 0.9 (0.9-1.0) | |
| Methemoglobin, % | Birth to <5 y | 90 | 0.48 (0.44-0.54) | 1.45 (1.36-1.53) |
| 5 to <19 y | 184 | 0.6 (0.6-0.7) | 1.1 (1.0-1.2) | |
| Potassium, mmol/L | Birth to <19 y | 275 | 3.5 (3.4-3.5) | 4.7 (4.6-4.7) |
| Chloride, mmol/L | Birth to <1 y | 40 | 96 (94-97) | 108 (107-109) |
| 1 to <5 y | 56 | 101 (100-102) | 111 (110-112) | |
| 5 to <19 y | 184 | 101 (101-102) | 107 (107-108) | |
| Sodium, mmol/L | Birth to <5 y | 81 | 135 (134-136) | 143 (142-144) |
| 5 to <19 y | 150 | 138 (137-139) | 143 (143-143) | |
| Ionized calcium, mmol/L | Birth to <19 y | 268 | 1.19 (1.19-1.19) | 1.33 (1.31-1.35) |
| Lactate, mmol/L | Birth to <19 y | 271 | 0.6 (0.6-0.7) | 2.1 (1.8-2.3) |
CALIPER, Canadian Laboratory Initiative on Paediatric Reference Intervals; CI, confidence interval; POCT, point-of-contact testing.
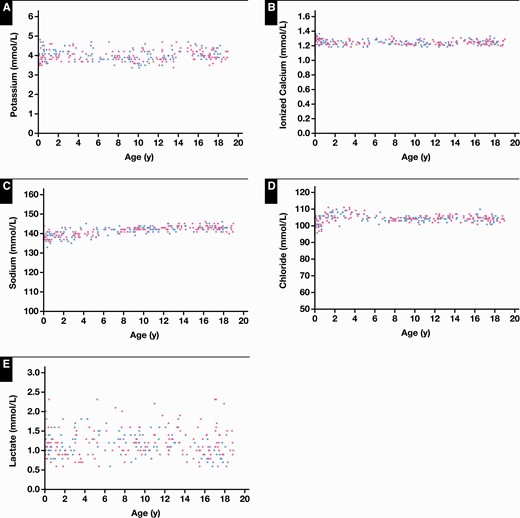
Reference value distributions for critical care whole blood biochemical parameters on the Radiometer ABL90 FLEX PLUS blood gas analyzer in the Canadian Laboratory Initiative on Paediatric Reference Intervals cohort of healthy children and adolescents, including potassium (A), ionized calcium (B), sodium (C), chloride (D), and lactate (E). Blue dots represent boys and pink dots represent girls.
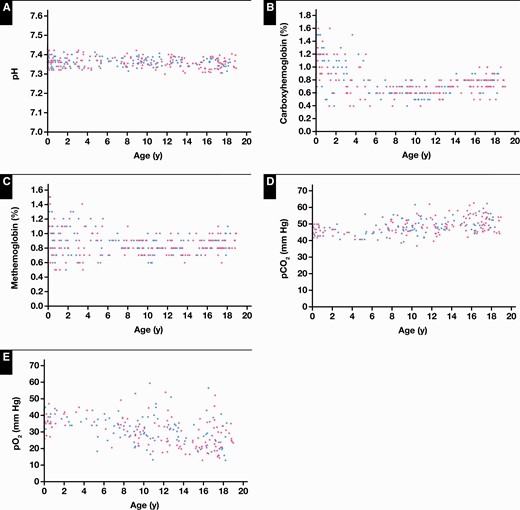
Reference value distributions for critical care whole blood biochemical parameters on the Radiometer ABL90 FLEX PLUS blood gas analyzer in the Canadian Laboratory Initiative on Paediatric Reference Intervals cohort of healthy children and adolescents, including pH (A), carboxyhemoglobin (B), methemoglobin (C), Pco2 (D), and Po2 (E). Blue dots represent boys and pink dots represent girls.
Reference value distributions for all electrolytes, including chloride, ionized calcium, sodium, and potassium, were consistent across the pediatric age range (Figure 1). Both potassium and ionized calcium demonstrated no age- or sex-specific differences. Thus, a single reference interval of birth to younger than 19 years of age was established for both parameters (Figures 1A and 1B). Sodium and chloride concentrations demonstrated some variation in early life. Specifically, reference value distributions for sodium were wider from birth to younger than 5 years of age and narrowed throughout the remainder of the pediatric age range, demonstrating statistically significant differences (Figure 1C). Chloride concentrations were significantly lower in the neonatal and infantile periods, increasing from 1 to younger than 5 years, and then remaining stable in later childhood and adolescence (Figure 1D). Similar to electrolytes, lactate demonstrated no statistically significant age- or sex-specific differences, although concentrations were fairly variable throughout the pediatric age range (Figure 1E).
As expected, pH demonstrated no statistically significant age- or sex-specific differences and could be described by 1 age partition (Figure 2A). Co-oximetry parameters, including carboxyhemoglobin and methemoglobin, required age partitioning in early life. Specifically, both carboxyhemoglobin and methemoglobin demonstrated increased levels from birth to younger than 5 years of age, decreasing to lower levels across the remainder of the pediatric age range (Figures 2B and 2C). Finally, unique reference value distributions were observed for Po2 and Pco2, demonstrating narrower distributions from birth to younger than 5 years of age, followed by wider variation in the early childhood and adolescent periods.
Discussion
There is an increasing trend toward the adoption of POCT systems in laboratory medicine, with demonstrated value in clinical care in hospitals, doctors’ offices, and remote health care settings. The clinical value of POCT systems, however, relies heavily on the availability of accurate reference standards for laboratory test interpretation and clinical decision-making. The current study evaluated 10 critical care parameters on the ABL90 FLEX PLUS blood gas analyzer in a large cohort of healthy children and adolescents, valuably contributing to our knowledge of normative pediatric values for critical care parameters on a commonly used POCT system.
As expected, most electrolytes demonstrated consistent reference value distributions throughout the pediatric age range, requiring few age-specific partitions and no sex-specific partitions (ie, sodium, potassium, chloride, ionized calcium). Most available normative data for electrolytes in the pediatric population are based on serum or plasma specimens, in contrast to the current study, which used whole blood. For example, the Canadian Health Measures Survey measured serum sodium, potassium, and chloride concentrations in thousands of healthy Canadian individuals aged 3 to 79 years on the VITROS 5600 FS analyzer.24 In this study, age- and sex-specific differences were noted for chloride and sodium,24 but reported upper and lower reference limits were similar across partitions, and observed statistical significance was likely the result of large sample size.24 Further, the Harmonizing Age Pathology Parameters in Kids initiative recently established direct pediatric continuous reference curves (30 days to 19 years) for serum electrolytes.25 Zierk and colleagues have also established continuous RI curves for plasma electrolytes using patient data and indirect statistical methodologies.26 These data also showed higher variation in the neonatal infantile period compared with later childhood and adolescence. Additionally, established quantitative limits in this study are similar to what is generally accepted in adults in serum and plasma, demonstrating slightly narrower distributions, likely because of the stringent exclusion criteria applied. In addition to reference standard studies, some literature has compared electrolyte measurements between POCT and laboratory instruments, with largely conflicting results.27-29 Unfortunately, the vast majority of these studies compare arterial whole blood measured on a POCT system to arterial or venous serum measured on a laboratory instrument. While some studies report good concordance in electrolyte results between whole blood and plasma/serum matrices, others found that the bias between measurements exceeded the acceptable limits of the Clinical Laboratory Improvement Amendment guidelines and thus are not interchangeable.27-29 Importantly, laboratories should verify reference standards according to CLSI guidelines for all intended sample matrices and platforms prior to implementation.
In addition to chloride, potassium, and sodium, we measured ionized calcium and lactate. It is well known that total calcium concentration is not an ideal marker of calcium homeostasis, with ionized calcium arguably being more physiologically relevant.30 Despite its increasing importance in calcium status assessment, there are minimal reference value data for ionized calcium in the literature for either pediatric or adult populations. The adult reference standard listed in the Tietz textbook for this parameter is 1.18 to 1.30 mmol/L.31 This value aligns well with our reported reference standard of 1.19 to 1.33 mmol/L, suggesting comparability between adult and pediatric values and justifying common ranges. Similar to ionized calcium, established reference standards for lactate confirm the applicability of previously reported data in adults. In this study, the majority of lactate concentrations were below 2.0 mmol/L, which is commonly the upper reference limit reported for venous lactate in clinical laboratories.32
Additional parameters evaluated in this study include pH, carboxyhemoglobin, and methemoglobin. Quantitative lower and upper reference limits mirrored what has previously been reported in textbooks and older literature. Specifically, the reference interval for venous pH has long been recognized as 7.31 to 7.41 or a minor variation thereof.31 Our results confirm this range exactly in the CALIPER cohort of healthy children and adolescents, suggesting that historic ranges remain valid for their application in pediatric test interpretation. Additionally, as carboxyhemoglobin is a complex of carbon monoxide that forms in RBCs when carbon monoxide is inhaled, it is expected that carboxyhemoglobin levels in the pediatric population should be low or nonexistent. There is currently no standardized cutoff, but laboratories commonly report less than 2% to 3% for nonsmoking individuals.33 In this study, all participant levels were less than 2%, and most values from participants aged 5 to younger than 19 years were less than 1%, as expected. Similarly, methemoglobin, a form of hemoglobin that cannot bind oxygen, should be low in healthy children and adolescents. In the current study, all participant levels were less than 1.5%. Slightly elevated levels of both carboxyhemoglobin and methemoglobin in early life may be physiologically explained by higher respiratory stress, although this has not been extensively reported in the literature, or potential underlying medical conditions from outpatient participants, including neonatal hyperbilirubinemia.34
Finally, given its status as one of the most routinely ordered laboratory panels, there are surprisingly few reference standard studies for blood gases in pediatric populations. One recent study by Klæstrup and colleagues35 established reference standards for blood gases on the Radiometer ABL725/835 analyzer in a cohort of healthy volunteers (aged 22-32 years), although most available data concern arterial blood gases. Arterial blood gas analysis has long been considered standard for evaluating Po2 and Pco2, but implementation of peripheral venous blood gas analysis is increasing, particularly in outpatient settings, because of the risks associated with arterial blood collection.36 In the current study, established reference standards for Pco2 were 40 to 51 mm Hg for participants aged birth to younger than 5 years and 41 to 61 mm Hg for participants aged 5 to younger than 19 years. Established reference standards for Po2 were 25 to 49 mm Hg for participants aged birth to younger than 5 years and 15 to 52 mm Hg for participants aged 5 to younger than 19 years. Traditionally, reference standards reported for venous blood gases in clinical laboratories were 41 to 51 mm Hg for Pco2 and 30 to 40 mm Hg for Po2.37 Increased variation in data observed in this study may represent novel physiologic trends or be the result of preanalytical factors encountered in community sampling. For Po2, however, it is unlikely that any aerobic exposure occurred because reference values decreased in relation to previously reported data and did not approach ambient pressure of 150 mm Hg. Although it is possible that observed decreases in Po2 and increases in Pco2 may be the result of ongoing in vitro glycolysis,38 samples were analyzed within 30 minutes of collection, thus limiting this preanalytical possibility. Variation in the time to analysis (ie, from within 1 to 30 minutes of collection) may account for some of the variability observed.
Pediatric venous whole blood reference standards for critical parameters on POCT systems have been seriously lacking in pediatric populations. The current study establishes reference standards for 10 analytes on the Radiometer ABL90 FLEX PLUS blood gas analyzer in the CALIPER cohort of healthy children and adolescents for the first time. This work will help facilitate the accurate interpretation of critical care test results on laboratory platforms with emerging clinical prominence and utility. Before implementation of these reference standards, it is important that they be verified for each laboratory’s analytical platform, intended sample matrices, and local pediatric population, as recommended by the CLSI EP28-A3c guidelines. Future work will focus on expanding the utility of the CALIPER database to other POCT systems, including those that incorporate chemistry and other specialized tests.
Acknowledgments
We thank all CALIPER participants and families, without whom this work would not have been possible. We also thank Radiometer Medical (Canada) for supplying the analytical instrument and all necessary reagents.
Funding
This work was supported by a Canadian Institute for Health Research (CIHR) Foundation Grant to K.A. (grant No. 353989) and a CIHR Doctoral Award awarded to M.K.B.
References
Rifai N, Wittwer C, Horvath AR, eds.




