-
PDF
- Split View
-
Views
-
Cite
Cite
Phillip D McMullen, Vera Tesic, Peter Pytel, Printculture of Surgical Pathology and Autopsy Specimens: A Comparison to Standard Culture Techniques, American Journal of Clinical Pathology, Volume 152, Issue 6, December 2019, Pages 747–756, https://doi.org/10.1093/ajcp/aqz090
Close - Share Icon Share
Abstract
Printculture is a method of microbiologic assessment previously described for use in the autopsy setting. We sought to compare printculture of surgical and autopsy pathology specimens to standard microbiology culture using matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF)–based colony identification.
Printculture was performed on 18 frozen samples with corresponding standard culture results. The results of MALDI-TOF identification of colonies recovered by printculture were compared with standard cultures, and percent concordance was calculated.
There was 95.8% concordance to standard culture methods for cases with infections and 100% concordance for cases without infection. The pattern of growth was found to aid in the distinction between contamination and true infection.
Printculture allows the identification of microorganisms from routinely frozen tissues and provides a bridge between microbiology and histomorphology through the identification of associated histologic features of infection. This technique can be successfully integrated into autopsy and surgical pathology workup of potentially infected tissues.
Diagnosis of an infection often requires demonstrating the presence of pathogenic organisms, either through culture or molecular techniques.1 While infections and reactive changes are not infrequently encountered in anatomic pathology, the overwhelming majority of microbiologic diagnosis is performed in a dedicated microbiology laboratory. The ability to provide useful diagnostic information relating infectious agents is somewhat limited in routine anatomic pathology practice. Immunohistochemical stains targeting specific pathogens such as Helicobacter pylori and Toxoplasma spp are commercially available, but most immunohistochemistry (IHC) probes target only specific pathogens, and the library of IHC probes targeting infectious agents is limited at most institutions.2,3 Next-generation sequencing of formalin-fixed, paraffin-embedded tissue for pathogen identification has been used with success, but it only is available at a select number of institutions, is relatively expensive, and has a longer turnaround time compared with standard culture techniques.4,5 A rapid, efficient means of processing standard anatomic pathology specimens for microbiologic analysis would therefore be of value.
Microbiologic assessment of anatomic pathology specimens itself may be regarded as conceptually problematic. The workplaces in anatomic pathology (grossing facilities and morgues) are considered nonsterile, contaminated spaces not ideal for standard microbiologic methods. Under ideal circumstances, cultures are submitted separately to the microbiology laboratory directly from the operating room. In cases of intraoperative consultation where infection is suspected but cultures have not been sent before interpretation of the frozen section, the surgeon may be advised to submit additional tissue for culture. Separately submitted cultures can, however, be discordant from the findings in surgical pathology material, not only in our own clinical experience and but also in prior studies.6 In addition, in some of these cases in which a frozen section unexpectedly shows features suggestive of an infection, submission of meaningful standard microbiology cultures from the operating room may be no longer possible due to limited material. In the autopsy suite, contamination, postmortem bacterial expansion, and tissue processing can make cultures difficult to interpret or impossible to perform.7 Therefore, the workflows of anatomic pathology and microbiology are generally independent, and there is often a separation between anatomic pathology and clinical microbiology. This separation becomes most obvious in cases with discordant results—cases in which organisms or changes associated with infections may be in the anatomic pathology specimen, but the cultures are negative, or vice versa.6
In the early 1970s, a protocol was developed that sought to provide microbiologic information from anatomic specimens in the setting of frozen section, known as printculture.8 The protocol is essentially a frozen section combined with standard microbiologic culture methods using the cut frozen section as the inoculum for culture.8-10 One proposed benefit of the method is the ability to discriminate surface contamination from organisms present in the tissue. Organisms considered true infections tend to grow within the confines of the tissue section, whereas contaminating microorganisms are limited to the area outside of the tissue, including the tissue surface.8-10 This observation was of particular utility in the setting of autopsy, where contamination is expected to be a frequent occurrence.7
While the printculture method was described independently in the literature three times, it remains largely unused and is unknown to most pathologists in both anatomic and clinical pathology. The studies detailing printculture demonstrated the ability of the method to provide interpretable colony morphologies from infected tissue sites, but a formal comparison to standard culture methods using prospective specimens has, to our knowledge, not been performed.8-10 In addition, matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF)–based identification of bacterial and fungal organisms is now available to rapidly and accurately compare printculture results with traditional culture methods. Herein, we describe our experience with printculture in both surgical and autopsy pathology specimens. In our experience, the method is able to identify the predominant pathogens in an infectious focus while also discriminating surface/procedural contamination, making it a useful method that bridges the laboratories of anatomic pathology and microbiology.
Materials and Methods
An institutional review board–approved prospective study was performed on both surgical and autopsy pathology specimens received at the University of Chicago Medical Center from March 2017 to April 2019. The main focus was to assess the validity of printculture results in the setting of autopsy, intraoperative consultation, and in the analysis of routine surgical specimens such as amputations. The results of printculture were compared with standard culture techniques, which at our institution consist of tissue homogenization and culture. MALDI-TOF–based identification of isolated colonies was used for both standard cultures and printcultures. Cases were selected based on the clinical suspicion of infection, either in the clinical history or based on the findings of frozen section or autopsy. In all cases except one, the standard cultures were collected at the same time as the printculture. In the exception case (an amputation), standard cultures were taken before antibiotic therapy, whereas the printculture was performed on the specimen after antibiotic therapy and amputation. For all cases analyzed, the sections were taken only after all other diagnostic sections were secured as to not compromise formal diagnosis.
After identifying candidate tissue to be sampled, a standard frozen section of the tissue was performed on a cryostat that had been sanitized by our standard cleaning protocol (ie, not sterilized). A pair of tweezers and an embedding spatula were cleaned using a bleach-based solution before tissue embedding. The tweezers were placed in the cryostat to cool, and the candidate tissue was embedded in a generous amount of optimal cutting temperature freezing medium at –20°C in the cryostat. Serial sections were performed until the tissue was fully faced, and then a standard 5-μm-thick section was taken and stained with H&E for histologic analysis. If tissue suspicious for an infection was identified on the H&E section, a separate 20- to 30-μm-thick section of tissue was performed. This thick section was placed evenly on microbiologic media using the chilled forceps to grab a corner of the section distant from the tissue Figure 1. Our initial panel of media comprised trypticase soy agar with 5% sheep blood agar, chocolate agar, Brucella blood agar with hemin and vitamin K (for anaerobic organisms), and Sabouraud dextrose agar plate (for fungal organisms) (BD Diagnostic Systems). Two thick sections of tissue were placed on each plate, and the plated specimens were transported to Clinical Microbiology for incubation in the appropriate incubators and subsequent identification.
![Schematic representation of the workflow of printculture and possible results. First the tissue is frozen in freezing media (optimal cutting temperature [OCT]) and then sectioned at 20 µm. The cut section is then grabbed by the freezing media, distant from the tissue section, and placed en face onto microbiologic media. Three patterns of growth are demonstrated: no growth (indicative of no infection or uncultivable organisms), media-only growth (consistent with contamination), and tissue/media growth (indicative of a true infection).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ajcp/152/6/10.1093_ajcp_aqz090/1/m_aqz090f0001.jpeg?Expires=1750393347&Signature=28XLvUWfTOX0sufnwijB9mRo~yfq~w1XlwTTpEwtDfxxPJ1yJVGqZkQNI1aSYY6BW4Ui7m8qLpecfkKpKvKVKLlPgQPFu~RVreabKsVtYr3Ks5I-ljEZufgyBhrQBzyMOvByZ5k9uLGBefsyVjR8ZlolkDVwl6UuRI6Sv2RZSuO~avQOeJNdobHFBHWqxNQBCRhiaXExZ2h-K5Y5vTmLeaxcEdAH4XF78UyQm8Xrpjd2gFW-x~M1f-j0ckKdAgOvzkklDC-Tr4t9GtOQUsg7gFC0Qe7vgpC3s9SN62btclSQekx3-l5j~fBcapyoBYixnPyilo5ghJ4o4sMdopGH7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Schematic representation of the workflow of printculture and possible results. First the tissue is frozen in freezing media (optimal cutting temperature [OCT]) and then sectioned at 20 µm. The cut section is then grabbed by the freezing media, distant from the tissue section, and placed en face onto microbiologic media. Three patterns of growth are demonstrated: no growth (indicative of no infection or uncultivable organisms), media-only growth (consistent with contamination), and tissue/media growth (indicative of a true infection).
The media plates were analyzed on a daily basis, and the location of any colonies was noted by taking photographs of the agar plates before subculture for MALDI-TOF analysis. The individual colonies were then identified using MALDI-TOF (Vitek MS, bioMérieux), and the species-level identifications were compared with the final report of the corresponding standard microbiology cultures for the specimen in question. The distribution of colonies on the tissue section was compared with the histologic sections and in some cases could be mapped directly onto the H&E-stained slide. The results were analyzed by calculating percent concordance for both negative and positive cases. Concordance was determined by comparing the number of speciated organisms present in the standard cultures with those recovered in the printculture specimens. For instance, if standard cultures identified three pathogens that were speciated, but printculture only detected two of those three, concordance would be 66%. If all three were detected by printculture, concordance would be 100%. No penalty was given for unspeciated mixed populations if such a population was not recovered by printculture. For instance, if printculture detected two pathogens, and the standard cultures detected two pathogens and a background of mixed, unspeciated microorganisms, concordance was still considered 100% since printculture was able to accurately recapitulate the speciated pathogens present in the tissue.
One theoretical concern with the printculture method is contamination, either from the nature of specimen collection (surgical and autopsy specimens received in the gross room or morgue) or by transferring organisms from the unsterilized cryostat onto the tissue. To interrogate the potential for contaminants, we analyzed the contaminants identified for each specimen and compared the speciated contaminants with organisms recovered from samples processed before the contaminated plate. We also sought to investigate if the frozen remnant kept at –80°C could still be used for future printculture analysis after long-term storage. The frozen remnants of 11 cases were reprocessed in an identical manner as during the initial plating. Sections were plated at intervals ranging from 3 months to 1 year after initial evaluation to interrogate the viability of microorganisms in the frozen remnant.
Results
Eighteen specimens were evaluated using printculture and standard culture methods. Thirteen of the cases were collected at autopsy, while five were surgical pathology specimens. All of the autopsy specimens were portions of lung, except for two samples of pectoralis muscle taken as negative controls. The surgical pathology specimens consisted of two bone biopsy specimens, one joint space lesion, one skin lesion, and one hip mass.
Cases Negative for Infection
Nine of the cases examined were called negative/indefinite for infection Table 1. Seven of these nine cases were negative by standard culture methods, and two exhibited growth of microorganisms by standard culture. Three of the seven specimens called negative by standard cultures exhibited scant growth of microorganisms in the media compartment on the printculture plates (areas of the plate not covered by the tissue section) Image 1. This is a pattern we and previous authors consider consistent with contamination. None of these seven plates exhibited growth of organisms within the tissue section of the printculture plate. Histologically, none of the completely negative cases exhibited pathologic changes that could be suggestive of infection. Overall, all seven cases truly negative for microbial growth by standard cultures were called negative for infection by printculture, representing 100% culture and diagnostic concordance. Two specimens called negative for infection by printculture did exhibit heavy growth of microorganisms in both standard cultures and printculture. Printculture plates for these two cases revealed distinct patterns of growth: diffuse polymicrobial growth through the tissue section and media compartment (PC6) and heavy surface growth with minimal tissue compartment growth (PC20). Specimen PC6 was obtained from a decedent with a long postmortem interval (>72 hours), and many of the colonies recovered were consistent with normal pulmonary flora, while others were consistent with gastrointestinal (GI) flora. Histologically, this case exhibited pulmonary edema and autolysis, but there was no evidence of inflammation. Thus, PC6 was favored to represent postmortem expansion of normal pulmonary and GI flora in the setting of putrefaction. In this case, histology combined with knowledge of normal flora components provided resolution.
Specimen PC20 was obtained from a surgical resection of a hip mass, which histologically was consistent with a high-grade sarcoma. The printculture plate for this specimen exhibited heavy polymicrobial growth predominantly on the tissue surface, with two colonies present in the tissue section. The tissue colonies in this case were favored to represent contaminants introduced into the tissue compartment by the microtome sectioning process, rather than a true infection, given the overall distribution of colonies. Upon further review of the patient’s medical record, however, it was found that the initial biopsy specimen of the sarcoma was complicated by a wound infection. It is possible, then, that the heavy “contamination” seen in this specimen was actually the result of a wound infection secondary to the patient’s biopsy specimen.
| Specimen . | Source . | Printculture Colonies . | Standard Culture . | Histology . | Culture Concordance, % . |
|---|---|---|---|---|---|
| PC2A | Autopsy, lung | Rare surface contaminants (Micrococcus luteus) | No growth | Normal lung | 100 |
| PC3A | Autopsy, lung | Rare surface contaminants (Streptococcus agalactiae) | No growth | ARDS | 100 |
| PC3B | Autopsy, lung | Rare surface contaminants (Streptococcus agalactiae) | No growth | ARDS | 100 |
| PC6 | Autopsy, lung | Diffuse growth of Candida tropicalis, Saccharomyces cerevisiae, Lactobacillus, "gram-negative rod," Escherichia coli, M luteus | C tropicalis, S cerevisiae, four or more aerobic gram-positive and gram- negative organisms | Pulmonary edema, autolysis, long postmortem interval with bacterial expansion | 100 |
| PC15 | Surgical pathology, bone | No growth | No growth | Rosai-Dorfman disease | 100 |
| PC17 | Surgical pathology, joint space | No growth | No growth | Fibrous tissue, chronic inflammation | 100 |
| PC18A | Autopsy, pectoralis muscle | No growth | No growth | Viable skeletal muscle | 100 |
| PC18B | Autopsy, pectoralis muscle | No growth | No growth | Viable skeletal muscle | 100 |
| PC20 | Surgical pathology, hip mass | Heavy surface contaminants with isolated tissue colonies (Klebsiella oxytoca, Enterobacter cloacae complex, Pseudomonas putida) | K oxytoca, E cloacae complex, P putida | High-grade sarcoma | 100 |
| Specimen . | Source . | Printculture Colonies . | Standard Culture . | Histology . | Culture Concordance, % . |
|---|---|---|---|---|---|
| PC2A | Autopsy, lung | Rare surface contaminants (Micrococcus luteus) | No growth | Normal lung | 100 |
| PC3A | Autopsy, lung | Rare surface contaminants (Streptococcus agalactiae) | No growth | ARDS | 100 |
| PC3B | Autopsy, lung | Rare surface contaminants (Streptococcus agalactiae) | No growth | ARDS | 100 |
| PC6 | Autopsy, lung | Diffuse growth of Candida tropicalis, Saccharomyces cerevisiae, Lactobacillus, "gram-negative rod," Escherichia coli, M luteus | C tropicalis, S cerevisiae, four or more aerobic gram-positive and gram- negative organisms | Pulmonary edema, autolysis, long postmortem interval with bacterial expansion | 100 |
| PC15 | Surgical pathology, bone | No growth | No growth | Rosai-Dorfman disease | 100 |
| PC17 | Surgical pathology, joint space | No growth | No growth | Fibrous tissue, chronic inflammation | 100 |
| PC18A | Autopsy, pectoralis muscle | No growth | No growth | Viable skeletal muscle | 100 |
| PC18B | Autopsy, pectoralis muscle | No growth | No growth | Viable skeletal muscle | 100 |
| PC20 | Surgical pathology, hip mass | Heavy surface contaminants with isolated tissue colonies (Klebsiella oxytoca, Enterobacter cloacae complex, Pseudomonas putida) | K oxytoca, E cloacae complex, P putida | High-grade sarcoma | 100 |
ARDS, acute respiratory distress syndrome.
| Specimen . | Source . | Printculture Colonies . | Standard Culture . | Histology . | Culture Concordance, % . |
|---|---|---|---|---|---|
| PC2A | Autopsy, lung | Rare surface contaminants (Micrococcus luteus) | No growth | Normal lung | 100 |
| PC3A | Autopsy, lung | Rare surface contaminants (Streptococcus agalactiae) | No growth | ARDS | 100 |
| PC3B | Autopsy, lung | Rare surface contaminants (Streptococcus agalactiae) | No growth | ARDS | 100 |
| PC6 | Autopsy, lung | Diffuse growth of Candida tropicalis, Saccharomyces cerevisiae, Lactobacillus, "gram-negative rod," Escherichia coli, M luteus | C tropicalis, S cerevisiae, four or more aerobic gram-positive and gram- negative organisms | Pulmonary edema, autolysis, long postmortem interval with bacterial expansion | 100 |
| PC15 | Surgical pathology, bone | No growth | No growth | Rosai-Dorfman disease | 100 |
| PC17 | Surgical pathology, joint space | No growth | No growth | Fibrous tissue, chronic inflammation | 100 |
| PC18A | Autopsy, pectoralis muscle | No growth | No growth | Viable skeletal muscle | 100 |
| PC18B | Autopsy, pectoralis muscle | No growth | No growth | Viable skeletal muscle | 100 |
| PC20 | Surgical pathology, hip mass | Heavy surface contaminants with isolated tissue colonies (Klebsiella oxytoca, Enterobacter cloacae complex, Pseudomonas putida) | K oxytoca, E cloacae complex, P putida | High-grade sarcoma | 100 |
| Specimen . | Source . | Printculture Colonies . | Standard Culture . | Histology . | Culture Concordance, % . |
|---|---|---|---|---|---|
| PC2A | Autopsy, lung | Rare surface contaminants (Micrococcus luteus) | No growth | Normal lung | 100 |
| PC3A | Autopsy, lung | Rare surface contaminants (Streptococcus agalactiae) | No growth | ARDS | 100 |
| PC3B | Autopsy, lung | Rare surface contaminants (Streptococcus agalactiae) | No growth | ARDS | 100 |
| PC6 | Autopsy, lung | Diffuse growth of Candida tropicalis, Saccharomyces cerevisiae, Lactobacillus, "gram-negative rod," Escherichia coli, M luteus | C tropicalis, S cerevisiae, four or more aerobic gram-positive and gram- negative organisms | Pulmonary edema, autolysis, long postmortem interval with bacterial expansion | 100 |
| PC15 | Surgical pathology, bone | No growth | No growth | Rosai-Dorfman disease | 100 |
| PC17 | Surgical pathology, joint space | No growth | No growth | Fibrous tissue, chronic inflammation | 100 |
| PC18A | Autopsy, pectoralis muscle | No growth | No growth | Viable skeletal muscle | 100 |
| PC18B | Autopsy, pectoralis muscle | No growth | No growth | Viable skeletal muscle | 100 |
| PC20 | Surgical pathology, hip mass | Heavy surface contaminants with isolated tissue colonies (Klebsiella oxytoca, Enterobacter cloacae complex, Pseudomonas putida) | K oxytoca, E cloacae complex, P putida | High-grade sarcoma | 100 |
ARDS, acute respiratory distress syndrome.
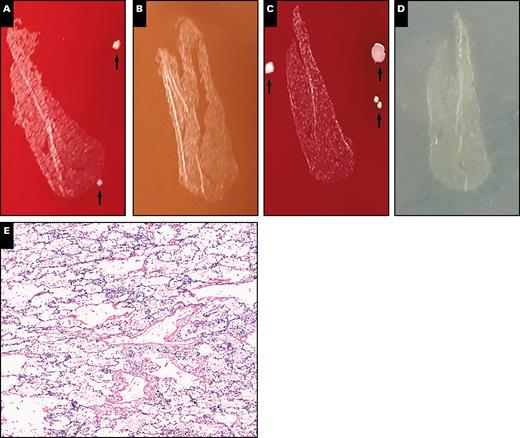
Representative plate photos and histology of cases called negative for infection (case PC3B). A-D, Isolated, rare colonies are present in the media section and on the tissue surface (arrows) but are not present in the tissue section itself. A, Blood. B, Chocolate agar. C, Anaerobic. D, Fungal. E, Histologically bland-appearing lung parenchyma without evidence of inflammation is demonstrated (x10).
Cases Positive for Infection
The remaining nine cases were all considered positive for infection, and microorganisms were isolated by standard culture methods for each of these specimens Table 2. Five (55.6%) of these nine cases were mixed infections, where the standard cultures reported up to four predominant pathogens in a background of mixed gram-positive and gram-negative organisms. The remainder of positive cases (n = 4, 44.4%) showed growth of only a single dominant organism. Printculture successfully recovered the predominant pathogen in all cases of single-organism growth, including one case of invasive aspergillosis caused by Aspergillus fumigatus. Histologic evaluation of these cases revealed findings suggestive of infection, including acute inflammation, necrosis, or microorganisms. Representative plate photos and histology of select single-organism positive cases are shown in Image 2.
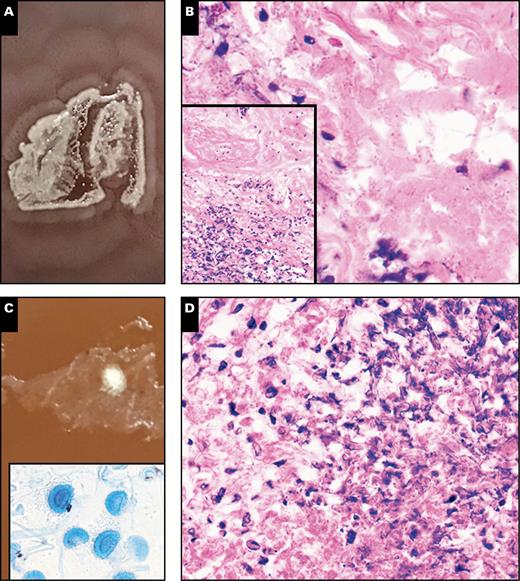
Representative photos of two cases of infections caused by single organisms (A and B, case PC13; C and D, case PC8B). A, A plate photo demonstrating heavy growth of Proteus mirabilis on chocolate agar, with a classic swarming motility pattern of growth. B, High-power (x40) histologic view of microorganisms at an interface of necrotic and viable tissue within the tissue section (inset, low power (x10) view of interface). C, Plate photo demonstrating a single filamentous colony, which upon scotch-tape preparation and staining with lactophenol-cotton-blue demonstrated fruiting bodies consistent with Aspergillus fumigatus (inset) (x40). D, High-power (x40) histologic view of the area where the filamentous colony grew, demonstrating hyphal forms and necrosis, consistent with an invasive fungal infection.
In mixed infections, printculture was 88.2% concordant with the predominant pathogens listed by standard culture results. Two of the five mixed infection specimens were partially discordant. The first discordant specimen came from the lower lung lobe of a patient who died of complications of aspiration pneumonia. Four predominant organisms were reported in the standard cultures (Pseudomonas aeruginosa, Enterococcus faecalis, Candida albicans, and Candida glabrata), in a background of mixed unspeciated (likely nonpathogenic) gram-positive and gram-negative bacterial organisms. Printculture detected three of the four reported pathogens but did not recover C glabrata, resulting in 75% culture concordance Table 2. The other partially discordant specimen was an amputation specimen received in surgical pathology (mentioned above as the only specimen in our series where the standard cultures were taken separately from printculture). The standard cultures grew Staphylococcus aureus, Enterococcus faecalis, and Pseudomonas aeruginosa. Printculture failed to detect E faecalis in this specimen, representing 66% culture concordance. In this case, the wound cultures were performed before administration of daptomycin (to which the strain of E faecalis was susceptible), whereas the printculture of the amputation was performed following therapy. The discordance in this case is therefore likely the result of antibiotic therapy.
| Specimen . | Source . | Printculture Colonies . | Standard Culture . | Histology . | Culture Concordance, % . |
|---|---|---|---|---|---|
| PC4 | Autopsy, lung | Candida glabrata, Candida albicans | C glabrata, C albicans, four or more aerobic gram-positive organisms present | Acute aspiration, yeast present | 100 |
| PC5A | Autopsy, lung | Pseudomonas aeruginosa, Streptococcus anginosis, Streptococcus constellatus, Staphylococcus lugdunensis, C glabrata, Candida krusei | P aeruginosa and four or more strains of aerobic gram-positive and gram-negative organisms (fungal cultures not performed) | Acute pneumonia | 100 |
| PC7F | Autopsy, lung | P aeruginosa, Enterococcus faecalis, C albicans, and Corynebacterium striatum | P aeruginosa, E faecalis, C albicans, C glabrata, and four or more aerobic gram-positive and gram-negative organisms | Necrotizing pneumonia | 75 |
| PC8B | Autopsy, lung | Aspergillus fumigatus | A fumigatus | Invasive aspergillosis | 100 |
| PC11Ca | Surgical pathology, skin | Staphylococcus aureus, P aeruginosa, C striatum, andStreptococcus stricatus | S aureus, E faecalis, P aeruginosa, four or more strains of gram- positive and gram- negative organisms | Gangrenous necrosis | 66 |
| PC13 | Autopsy, lung | Proteus mirabilis | P mirabilis | Bacterial organisms in pulmonary vessels and alveolated parenchyma with acute inflammation and necrosis; sepsis secondary to P mirabilis | 100 |
| PC14B | Autopsy, lung | Scattered growth of Candida lusitaniae in tissue, moderate P mirabilis and few P aeruginosa in media compartment | C lusitaniae | Necrotizing pneumonia | 100 |
| PC16 | Surgical pathology, bone | S aureus | S aureus | Osteomyelitis | 100 |
| PC19 | Autopsy, lung | E faecalis and Enterococcus faecium | E faecalis and E faecium | Exudative ARDS following aspiration | 100 |
| Specimen . | Source . | Printculture Colonies . | Standard Culture . | Histology . | Culture Concordance, % . |
|---|---|---|---|---|---|
| PC4 | Autopsy, lung | Candida glabrata, Candida albicans | C glabrata, C albicans, four or more aerobic gram-positive organisms present | Acute aspiration, yeast present | 100 |
| PC5A | Autopsy, lung | Pseudomonas aeruginosa, Streptococcus anginosis, Streptococcus constellatus, Staphylococcus lugdunensis, C glabrata, Candida krusei | P aeruginosa and four or more strains of aerobic gram-positive and gram-negative organisms (fungal cultures not performed) | Acute pneumonia | 100 |
| PC7F | Autopsy, lung | P aeruginosa, Enterococcus faecalis, C albicans, and Corynebacterium striatum | P aeruginosa, E faecalis, C albicans, C glabrata, and four or more aerobic gram-positive and gram-negative organisms | Necrotizing pneumonia | 75 |
| PC8B | Autopsy, lung | Aspergillus fumigatus | A fumigatus | Invasive aspergillosis | 100 |
| PC11Ca | Surgical pathology, skin | Staphylococcus aureus, P aeruginosa, C striatum, andStreptococcus stricatus | S aureus, E faecalis, P aeruginosa, four or more strains of gram- positive and gram- negative organisms | Gangrenous necrosis | 66 |
| PC13 | Autopsy, lung | Proteus mirabilis | P mirabilis | Bacterial organisms in pulmonary vessels and alveolated parenchyma with acute inflammation and necrosis; sepsis secondary to P mirabilis | 100 |
| PC14B | Autopsy, lung | Scattered growth of Candida lusitaniae in tissue, moderate P mirabilis and few P aeruginosa in media compartment | C lusitaniae | Necrotizing pneumonia | 100 |
| PC16 | Surgical pathology, bone | S aureus | S aureus | Osteomyelitis | 100 |
| PC19 | Autopsy, lung | E faecalis and Enterococcus faecium | E faecalis and E faecium | Exudative ARDS following aspiration | 100 |
ARDS, acute respiratory distress syndrome.
aThe standard cultures for this case were taken before treatment with daptomycin, whereas the printculture specimen was taken after treatment and amputation. The strain of E faecalis isolated in the standard cultures was susceptible to daptomycin.
| Specimen . | Source . | Printculture Colonies . | Standard Culture . | Histology . | Culture Concordance, % . |
|---|---|---|---|---|---|
| PC4 | Autopsy, lung | Candida glabrata, Candida albicans | C glabrata, C albicans, four or more aerobic gram-positive organisms present | Acute aspiration, yeast present | 100 |
| PC5A | Autopsy, lung | Pseudomonas aeruginosa, Streptococcus anginosis, Streptococcus constellatus, Staphylococcus lugdunensis, C glabrata, Candida krusei | P aeruginosa and four or more strains of aerobic gram-positive and gram-negative organisms (fungal cultures not performed) | Acute pneumonia | 100 |
| PC7F | Autopsy, lung | P aeruginosa, Enterococcus faecalis, C albicans, and Corynebacterium striatum | P aeruginosa, E faecalis, C albicans, C glabrata, and four or more aerobic gram-positive and gram-negative organisms | Necrotizing pneumonia | 75 |
| PC8B | Autopsy, lung | Aspergillus fumigatus | A fumigatus | Invasive aspergillosis | 100 |
| PC11Ca | Surgical pathology, skin | Staphylococcus aureus, P aeruginosa, C striatum, andStreptococcus stricatus | S aureus, E faecalis, P aeruginosa, four or more strains of gram- positive and gram- negative organisms | Gangrenous necrosis | 66 |
| PC13 | Autopsy, lung | Proteus mirabilis | P mirabilis | Bacterial organisms in pulmonary vessels and alveolated parenchyma with acute inflammation and necrosis; sepsis secondary to P mirabilis | 100 |
| PC14B | Autopsy, lung | Scattered growth of Candida lusitaniae in tissue, moderate P mirabilis and few P aeruginosa in media compartment | C lusitaniae | Necrotizing pneumonia | 100 |
| PC16 | Surgical pathology, bone | S aureus | S aureus | Osteomyelitis | 100 |
| PC19 | Autopsy, lung | E faecalis and Enterococcus faecium | E faecalis and E faecium | Exudative ARDS following aspiration | 100 |
| Specimen . | Source . | Printculture Colonies . | Standard Culture . | Histology . | Culture Concordance, % . |
|---|---|---|---|---|---|
| PC4 | Autopsy, lung | Candida glabrata, Candida albicans | C glabrata, C albicans, four or more aerobic gram-positive organisms present | Acute aspiration, yeast present | 100 |
| PC5A | Autopsy, lung | Pseudomonas aeruginosa, Streptococcus anginosis, Streptococcus constellatus, Staphylococcus lugdunensis, C glabrata, Candida krusei | P aeruginosa and four or more strains of aerobic gram-positive and gram-negative organisms (fungal cultures not performed) | Acute pneumonia | 100 |
| PC7F | Autopsy, lung | P aeruginosa, Enterococcus faecalis, C albicans, and Corynebacterium striatum | P aeruginosa, E faecalis, C albicans, C glabrata, and four or more aerobic gram-positive and gram-negative organisms | Necrotizing pneumonia | 75 |
| PC8B | Autopsy, lung | Aspergillus fumigatus | A fumigatus | Invasive aspergillosis | 100 |
| PC11Ca | Surgical pathology, skin | Staphylococcus aureus, P aeruginosa, C striatum, andStreptococcus stricatus | S aureus, E faecalis, P aeruginosa, four or more strains of gram- positive and gram- negative organisms | Gangrenous necrosis | 66 |
| PC13 | Autopsy, lung | Proteus mirabilis | P mirabilis | Bacterial organisms in pulmonary vessels and alveolated parenchyma with acute inflammation and necrosis; sepsis secondary to P mirabilis | 100 |
| PC14B | Autopsy, lung | Scattered growth of Candida lusitaniae in tissue, moderate P mirabilis and few P aeruginosa in media compartment | C lusitaniae | Necrotizing pneumonia | 100 |
| PC16 | Surgical pathology, bone | S aureus | S aureus | Osteomyelitis | 100 |
| PC19 | Autopsy, lung | E faecalis and Enterococcus faecium | E faecalis and E faecium | Exudative ARDS following aspiration | 100 |
ARDS, acute respiratory distress syndrome.
aThe standard cultures for this case were taken before treatment with daptomycin, whereas the printculture specimen was taken after treatment and amputation. The strain of E faecalis isolated in the standard cultures was susceptible to daptomycin.
One of the mixed infection specimens grew C glabrata and C albicans in a background of mixed organisms (four or more unspeciated gram-positive and gram-negative organisms). By printculture, however, only C glabrata and C albicans were recovered. This particular specimen was taken at autopsy in a patient with witnessed aspiration before death. The background unspeciated organisms present in the standard culture could have been the result of contamination or from sampling an area of lung containing aspirated gastric material in addition to the fungal organisms. Similar to cases with a single dominant pathogen, histopathologic evaluation for cases of mixed infections universally revealed findings supporting true infections. The overall concordance for predominant pathogen(s) was 95.8% among the positive cases, regardless of whether a single organism or multiple organisms were present. Representative plate photos and histology for mixed infections are shown in Image 3.
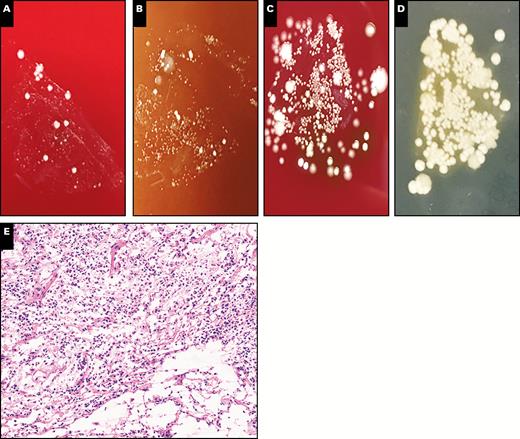
Representative plate photos and histology demonstrating a mixed infection (case PC5B). A-D, Different media demonstrated a variety of microbial colonies, including yeast colonies. A, Blood. B, Chocolate agar. C, Anaerobic. D, Fungal. E, Histologically, there is evidence of acute inflammation and tissue destruction, consistent with an active infection (x10). The patient had a history of aspiration pneumonia, which was confirmed both microbiologically and histologically using printculture.
Contamination of Specimens
Two specimens were found to exhibit significant contamination. One specimen (PC14B) grew a few colonies of Candida lusitaniae within the tissue section and in the standard microbiology cultures but exhibited moderate to heavy growth of Proteus mirabilis and rare colonies of P aeruginosa in the media compartment outside of the tissue section on printculture Image 4. Based on this pattern, the colonies in question were easily flagged as contamination on initial review of the plate. Review of the order of specimen processing revealed that two cases processed before this specimen contained heavy amounts of P aeruginosa (PC11C) and P mirabilis (PC13A). Thus, there appears to be the potential for heavily infected tissues to contaminate subsequent specimens, but the contamination is usually easily identifiable based on the location of the colonies on the agar plate, as previously described in the literature.8-10
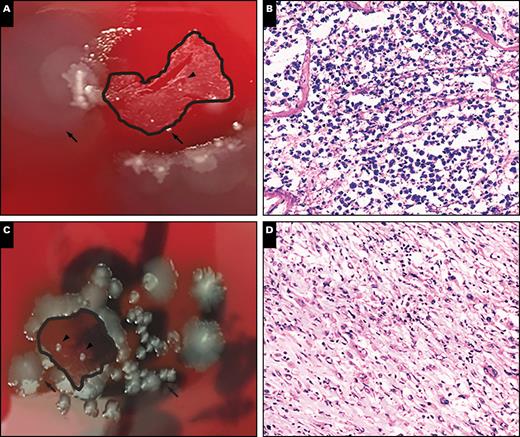
Representative plate photos and histology demonstrating the appearance of contamination (A and B, case PC14B; C and D, case PC20). A, Note the presence of colonies on the tissue surface (arrows), with scattered small colonies present in the tissue section (arrowheads). B, There is histologic evidence of acute inflammation, consistent with an active infection (x20). The colonies present within the tissue were speciated as Candida luscitaneae, which was consistent with premortem bronchoalveolar lavage cultures in this patient. The surface/media colonies were speciated as Proteus mirabilis and Pseudomonas aeruginosa, which were not recovered in the patient’s original premortem cultures. C, Dense surface growth (arrows), with two isolated colonies present in the tissue section (arrowheads). D, Histologic examination reveals a high-grade sarcoma favoring the tissue colonies to represent contamination from the surface (x10).
The second specimen with heavy contamination (PC20) exhibited heavy surface growth with minimal tissue growth. Histologic evaluation revealed a high-grade sarcoma with no definitive focus of infection. As mentioned above, the patient was treated for a wound infection at the time of his final resection, and thus these colonies could feasibly be the result of his known wound infection. Three organisms were reported in standard cultures submitted concurrently, with a 100% concordance between the standard cultures and printculture. Histologically, however, there was not sufficient evidence to call this case a true infection, even with isolated colonies in the tissue section (Image 4).
Maintenance of Frozen Tissue and Reproducibility of Results
The frozen remnants of 11 cases were replated at intervals ranging from 3 months to 1 year after initial evaluation to interrogate the stability of microorganisms in the frozen remnant. While there was a subtle loss in colony density, 10 (90.9%) of the 11 were concordant with the original printculture results. The exception case was PC8B, which initially recovered A fumigatus. The specimen was replated after approximately 1 year of storage in the –80°C freezer and failed to reproduce fungal colonies, even with multiple serial sections. The H&E-stained sections, however, demonstrated hyphal structures in an area of necrosis, consistent with invasive aspergillosis. The original specimen had been plated the same day as recovery, and only a single colony of filamentous fungus was present within the tissue section at that time. Thus, long-term storage of complex fungal organisms may not be possible by printculture, but otherwise, bacterial organisms appeared stable over long periods of freezing.
Discussion
Printculture results correlate very well with the results of standard microbiology cultures in our experience. Culture concordance for specimens with negative conventional cultures was 100%, and concordance for specimens with positive conventional cultures was 95.8%, regardless of whether a single organism or multiple organisms were recovered. Cases with only a single predominant pathogen were 100% culturally concordant with standard culture methods, while concordance for mixed infections was at 88.2%.
There are several benefits of the method. First and foremost, the ability to sample tissue directly from a specimen suspicious for infection allows a seamless and immediate integration of culture results and histomorphology. The method may be most useful in the intraoperative setting, where evidence of infection may be present on a frozen section. Additional material sampled by the surgeon in such instances may not necessarily be representative of the site that prompted evaluation by culture in the first place.6 Evaluation of such cases by standard methods could conceivably produce discordant results, in which histologically, an infection can easily be diagnosed but cultures remain negative. Also, printculture may be used in situations where tissue is limited or where standard culture was not performed for any reason.
One other benefit to the method is its relative ability to discriminate contamination based on the location of growth. A clean tissue section with no growth, or scattered colonies located outside of the section, was considered negative for infection (Image 1). Growth of microorganisms on the surface of the tissue section, without significant growth within the tissue section, was also considered likely contamination (Image 4). Colonies had to be convincingly present and abundant within the tissue section to be considered truly positive for infection. Discretion is required, however, as growth within the tissue section does not in and of itself indicate an infection is present. Normal flora can be recovered in high numbers depending on the sampled site, especially in autopsy specimens with long postmortem intervals, where bacterial expansion is expected (such as PC6). Thus, histologic correlation is an added benefit of the method and allows for more accurate interpretation of the culture results and their significance.
With regard to workflow, the freezing and sectioning processes of printculture are standard methods in anatomic pathology, which are used daily and could be easily incorporated into these laboratories. The fact that printculture has not been embraced more widely does not necessarily indicate the method is superfluous. Instead, we believe printculture could be easily integrated into the current workflow of surgical pathologists and autopsy pathologists. In addition, frozen-section specimens can still be analyzed by printculture even after an extended period of time in the freezer. The surgical pathologist could therefore consider keeping the frozen-section remnant frozen if the intraoperative assessment suggests the possibility of an infection. The decision to process the frozen tissue for routine histologic analysis could be delayed until the results of conventional microbiology cultures are available. The same is true for the autopsy setting, with the added benefit that the method appears to be capable of discriminating pathogens present in tissue from contaminants located on the tissue surface or outside of the tissue altogether.
In summary, printculture allows for accurate recovery of microorganisms from tissue sections and therefore represents integration between histomorphologic changes and microbiologic culture results. The ability to correlate histologic findings with culture results on a single piece of tissue is of great value. The importance of integrating histologic features and culture results was highlighted in a recent consensus statement on postmortem microbiology by the European Society of Clinical Microbiology and Infectious Diseases Study Group of Forensic and Postmortem Microbiology.11




