-
PDF
- Split View
-
Views
-
Cite
Cite
Huikang Xie, Hang Su, Donglai Chen, Dong Xie, Chenyang Dai, Yijiu Ren, Yunlang She, Long Xu, Shengnan Zhao, Chunyan Wu, Gening Jiang, Chang Chen, Use of Autofluorescence to Intraoperatively Diagnose Visceral Pleural Invasion From Frozen Sections in Patients With Lung Adenocarcinoma 2 cm or Less: A Prospective Study, American Journal of Clinical Pathology, Volume 152, Issue 5, November 2019, Pages 608–615, https://doi.org/10.1093/ajcp/aqz081
Close - Share Icon Share
Abstract
We prospectively investigate the accuracy of frozen sections for diagnosing visceral pleural invasion (VPI) by autofluorescence and evaluated its usefulness in sublobar resection.
We included patients with lung adenocarcinoma 2 cm or less to evaluate the diagnostic performance of autofluorescence for VPI in frozen sections via a fluorescence microscope. Furthermore, the impact of VPI on patients treated with sublobar resection was assessed in another cohort.
A total of 112 patients were enrolled. The accuracy, sensitivity, and specificity of autofluorescence for VPI diagnosis was 95.5%, 86.8%, and 100%, respectively. Sublobar resection was an independent risk factor for recurrence in patients with lung adenocarcinomas 2 cm or less with VPI positivity (hazard ratio, 3.30; P = .023), whereas it was not in those with VPI negativity.
Using autofluorescence in frozen sections appears to be an accurate method for diagnosing VPI, which is helpful for surgical decision making.
Recent studies have suggested that sublobar resection provides a similar oncologic outcome compared with lobectomy for small-sized (≤2 cm) lung adenocarcinoma.1,2 Meanwhile, data from the Surveillance, Epidemiology, and End Results (SEER) Program and the National Cancer Database (NCDB), as well as studies from Europe and east Asia, indicate that up to 20% to 30% of small-size invasive lung adenocarcinoma are treated by sublobar resection.3-6 Indeed, several independent predictors of recurrence after sublobar resection have been identified, including the presence of tumor spread through air spaces and visceral pleural invasion (VPI).7-9
VPI has been known to be a crucial adverse prognostic factor and patients may be considered candidates for more aggressive treatment.10-12 Currently, VPI is usually diagnosed based on postoperative elastic staining because of the unsatisfactory accuracy of preoperative imaging test.13 In the ongoing phase III randomized trials of lobectomy vs sublobar resection (CALBG 140503 and JCOG0802/WJOG4607L), the inclusion criteria restrict the tumor size to 2 cm or less. However, there were no criteria concerning VPI, because thoracic surgeons are unable to identify VPI until specimens are evaluated by pathologic examination; tumors with VPI will be upstaged to the next T category. Furthermore, VPI correlates with more frequent lymph node metastasis, especially skip N2 metastasis in small-size lung adenocarcinomas.10,14 Hence, for tumors 2 cm or less with VPI, the extent of resection should not be sublobar with insufficient lymph node dissection.
Recently, we have investigated a novel technique using autofluorescence to identify VPI in frozen sections during surgery. Autofluorescence offers a powerful resource for directly monitoring the biological substrate condition and does not require fixing or staining of the specimens.15 Intense autofluorescence can be observed in the visceral pleura under a fluorescence microscope because the surface structure of visceral pleura contains two or three layers of interwoven elastin fibers. This intrinsic biomarker may be useful for the diagnosis of VPI.15,16
In this study, we aimed to investigate the accuracy of frozen sections for diagnosing VPI by autofluorescence and the diagnostic reproducibility. We further evaluated the prognostic impact of VPI on patients with lung adenocarcinoma 2 cm or less treated with sublobar resection.
Material and Methods
Patient Selection
The Institutional Review Board of Shanghai Pulmonary Hospital approved this prospective study (No. K18-108). Patients who underwent surgery at our hospital from October 30, 2017, to December 15, 2017, were included in this study. The inclusion criteria consisted of three parameters: (1) tumor size 2.0 cm or less, (2) nodules with pleural indentation or pleural attachment or pleural proximity, and (3) pathologically confirmed invasive lung adenocarcinoma. Exclusion criteria are listed in Supplemental Figure 1 (all supplemental materials can be found at American Journal of Clinical Pathology online).
Radiological and Histopathologic Evaluation
Two reviewers (H.S. and D.C.) independently evaluated all computed tomography (CT) scans. The nodule pleural connections were stratified into three types in the lung window setting (level, –500 Hounsfield unit [HU]; width, 1,350 HU): (1) pleural indentation was defined as one or more linear pleural tags, (2) pleural attachment was defined as no visible space between the nodule and the visceral pleura on CT scans or tumor attachment to the interlobar pleura, and (3) pleural closeness was defined as a tumor located within 1.0 cm of the pleura. Two pathologists assessed the histologic subtypes of adenocarcinoma according to the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification.17
Intraoperatively Diagnosed VPI
Prosection, sampling, and staining were performed in a systematic manner and a flow chart of the procedures is shown in Figure 1. A fresh specimen was sliced at the largest diameter into block 1 and block 2 immediately after being removed by a thoracic surgeon, and these two blocks were used for intraoperative frozen pathology and final pathologic examination, respectively.
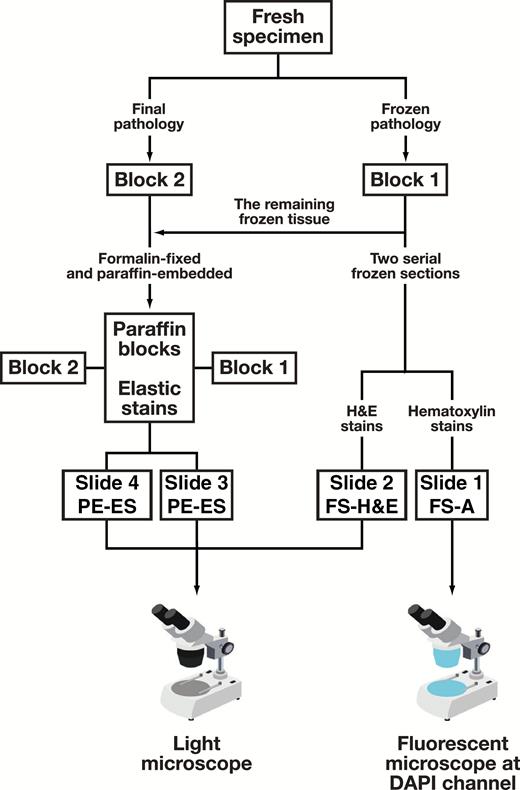
Flow chart of the procedures for diagnosis visceral pleural invasion. Slides 1 and 2 were cut from frozen tissue intraoperatively; slides 3 and 4 were cut from paraffin-embedded tissue. Slides 1, 2, and 3 were cut from the same block (block 1); slide 4 was cut from block 2. DAPI, 4′,6-diamidino-2-phenylindole; FS-A, frozen section autofluorescence; FS-H&E, frozen section H&E staining; PE-ES, paraffin-embedded tissue section elastic staining.
Two serial 4-μm thick sections were taken from the frozen tissue (block 1) without formalin perfusion fixation. The first slide was used for intraoperative frozen section autofluorescence (FS-A) diagnosis, which was carried out in three steps and took about 5 minutes (Figure 1): (1) hematoxylin staining for 1 minute; (2) rinsing, drying, mounting, and cover slip added; and (3) the first senior pathologist (H.X.) evaluated VPI using a fluorescence microscope set to the 4′,6-diamidino-2-phenylindole fluorescent dye channel (fluorescence excitation and emission maxima 350-420/420-510 nm). This channel was selected as it best displayed the elastic layer to identify the tumor cells, according to our investigations.
The second frozen section slide was simultaneous analyzed by routine H&S staining (FS-H&E), which was examined by the second senior pathologist using a light microscope. The second pathologist was blind to the FS-A results. Both FS-A and FS-H&E were intraoperatively classified as PL0, PL1, or PL2 according to the eighth TNM classification without deferral18: (1) PL0 is defined as lack of pleural invasion beyond the elastic layer; (2) PL1 is invasion beyond the elastic layer; and (3) PL2 is invasion to the surface of the visceral pleura. VPI includes PL1 and PL2.
Postoperatively Diagnosed VPI
Paraffin-embedded tissue elastic staining (PE-ES) was carried out on two slides from each patient. One slide was from frozen tissue (block 1), as a reference for the frozen section autofluorescence diagnosis, and the other was from block 2. The two slides were examined following the standard diagnostic method for VPI. Details of the elastic staining method have been described in a previous study.19 The third senior pathologist (C.W.), who was blind to the results of FS-A and FS-H&E diagnoses, evaluate VPI based on the elastic staining. VPI positivity included results from examination of tissue from either block 1 or block 2, or both.
Intraobserver and Interobserver Diagnostic Reproducibility Analysis
For intraobserver reproducibility, the first pathologist (H.X.) performed additional diagnosis of VPI for 50 cases that were randomly selected from the 112 cases 3 months after the initial diagnosis. For interobserver reproducibility, a fourth pathologist (S. Z.), blind to the previous diagnostic results, evaluated VPI based on FS-A for all the cases (n = 112).
Determination of Reasons for Misdiagnosis by Frozen Section Autofluorescence
To analyze the reasons for misdiagnoses, we used a frozen section control slide, which was cut from the paraffin block of the remaining frozen tissue (block 1) as a reference for the original frozen section. Frozen section control slides were prepared for elastic staining (PE-ES block 1). If the interpretation was the same for FS-A and PE-ES block 1, but different from that for PE-ES block 2, we considered the misdiagnosis was attributed to sampling error. If the interpretation was the same for PE-ES block 1 and PE-ES block 2, but different from that for FS-A, we considered the misdiagnosis was attributed to interpretation error.
Patient Follow-up
To evaluate the prognostic effect of VPI, consecutive patients with lung adenocarcinomas 2 cm or less who underwent surgery between 2009 and 2010 were identified for analysis. This time period was used because of the absence of survival data from patients who were treated between October 30, 2017, and December 15, 2017. Elastic staining was performed on recut slides from paraffin-embedded blocks for all these patients to confirm VPI. All patients were followed up from the day of surgery. Recurrence-free survival (RFS) was defined as the time from surgery until recurrence or death from any cause.
Statistical Analysis
We used Pearson χ2 test to compare categorical variables and an independent sample t test was used compared the continuous variables between different groups. κ coefficient was used to evaluate the degree of intraobserver and interobserver agreement. Log-rank test and Cox proportional hazard regression model were applied to evaluate predictive factors for RFS. Statistical analyses were performed in SPSS 22.0 (IBM).
Results
Baseline Information
A total of 112 patients were included in our study. Among them, 38 were VPI positive as confirmed by postoperative elastic staining examination. Their clinical characteristics are shown in Table 1. In this study, frozen section diagnoses of VPI were not applied to real-time therapeutic patient management.
| Characteristics . | Patients, No. (%) . |
|---|---|
| Age, y | |
| Median (range) | 63 (39-79) |
| ≤65 | 69 (61.6) |
| >65 | 43 (38.4) |
| Sex | |
| Male | 54 (48.2) |
| Female | 58 (51.8) |
| Smoking | |
| Nonsmoker | 95 (84.8) |
| Current or exsmoker | 17 (15.2) |
| CEA | |
| ≤5 ng/mL | 103 (92) |
| >5 ng/mL | 9 (8) |
| Surgical approach | |
| VATS | 108 (96.4) |
| Thoracotomy | 4 (3.6) |
| Operation | |
| Sublobar resection | 10 (8.9) |
| Lobectomy | 102 (91.1) |
| Tumor location | |
| Upper and middle lobar | 78 (69.6) |
| Lower lobar | 34 (30.4) |
| Pertaining pleura | |
| Pleural indentation | 63 (56.3) |
| Pleural attachment | 29 (25.9) |
| Pleural closeness | 20 (17.8) |
| Tumor size | |
| ≤1 cm | 35 (31.3) |
| 1-2 cm | 77 (68.7) |
| Predominant pattern | |
| Lepidic | 28 (25.0) |
| Acinar | 46 (41.1) |
| Papillary | 26 (23.2) |
| Micropapillary | 3 (2.7) |
| Solid | 9 (8) |
| Lymph node metastasis | |
| Negative | 104 (92.9) |
| Positive | 8 (7.1) |
| Characteristics . | Patients, No. (%) . |
|---|---|
| Age, y | |
| Median (range) | 63 (39-79) |
| ≤65 | 69 (61.6) |
| >65 | 43 (38.4) |
| Sex | |
| Male | 54 (48.2) |
| Female | 58 (51.8) |
| Smoking | |
| Nonsmoker | 95 (84.8) |
| Current or exsmoker | 17 (15.2) |
| CEA | |
| ≤5 ng/mL | 103 (92) |
| >5 ng/mL | 9 (8) |
| Surgical approach | |
| VATS | 108 (96.4) |
| Thoracotomy | 4 (3.6) |
| Operation | |
| Sublobar resection | 10 (8.9) |
| Lobectomy | 102 (91.1) |
| Tumor location | |
| Upper and middle lobar | 78 (69.6) |
| Lower lobar | 34 (30.4) |
| Pertaining pleura | |
| Pleural indentation | 63 (56.3) |
| Pleural attachment | 29 (25.9) |
| Pleural closeness | 20 (17.8) |
| Tumor size | |
| ≤1 cm | 35 (31.3) |
| 1-2 cm | 77 (68.7) |
| Predominant pattern | |
| Lepidic | 28 (25.0) |
| Acinar | 46 (41.1) |
| Papillary | 26 (23.2) |
| Micropapillary | 3 (2.7) |
| Solid | 9 (8) |
| Lymph node metastasis | |
| Negative | 104 (92.9) |
| Positive | 8 (7.1) |
CEA, carcinoembryonic antigen; VATS, video-assisted thoracic surgery.
| Characteristics . | Patients, No. (%) . |
|---|---|
| Age, y | |
| Median (range) | 63 (39-79) |
| ≤65 | 69 (61.6) |
| >65 | 43 (38.4) |
| Sex | |
| Male | 54 (48.2) |
| Female | 58 (51.8) |
| Smoking | |
| Nonsmoker | 95 (84.8) |
| Current or exsmoker | 17 (15.2) |
| CEA | |
| ≤5 ng/mL | 103 (92) |
| >5 ng/mL | 9 (8) |
| Surgical approach | |
| VATS | 108 (96.4) |
| Thoracotomy | 4 (3.6) |
| Operation | |
| Sublobar resection | 10 (8.9) |
| Lobectomy | 102 (91.1) |
| Tumor location | |
| Upper and middle lobar | 78 (69.6) |
| Lower lobar | 34 (30.4) |
| Pertaining pleura | |
| Pleural indentation | 63 (56.3) |
| Pleural attachment | 29 (25.9) |
| Pleural closeness | 20 (17.8) |
| Tumor size | |
| ≤1 cm | 35 (31.3) |
| 1-2 cm | 77 (68.7) |
| Predominant pattern | |
| Lepidic | 28 (25.0) |
| Acinar | 46 (41.1) |
| Papillary | 26 (23.2) |
| Micropapillary | 3 (2.7) |
| Solid | 9 (8) |
| Lymph node metastasis | |
| Negative | 104 (92.9) |
| Positive | 8 (7.1) |
| Characteristics . | Patients, No. (%) . |
|---|---|
| Age, y | |
| Median (range) | 63 (39-79) |
| ≤65 | 69 (61.6) |
| >65 | 43 (38.4) |
| Sex | |
| Male | 54 (48.2) |
| Female | 58 (51.8) |
| Smoking | |
| Nonsmoker | 95 (84.8) |
| Current or exsmoker | 17 (15.2) |
| CEA | |
| ≤5 ng/mL | 103 (92) |
| >5 ng/mL | 9 (8) |
| Surgical approach | |
| VATS | 108 (96.4) |
| Thoracotomy | 4 (3.6) |
| Operation | |
| Sublobar resection | 10 (8.9) |
| Lobectomy | 102 (91.1) |
| Tumor location | |
| Upper and middle lobar | 78 (69.6) |
| Lower lobar | 34 (30.4) |
| Pertaining pleura | |
| Pleural indentation | 63 (56.3) |
| Pleural attachment | 29 (25.9) |
| Pleural closeness | 20 (17.8) |
| Tumor size | |
| ≤1 cm | 35 (31.3) |
| 1-2 cm | 77 (68.7) |
| Predominant pattern | |
| Lepidic | 28 (25.0) |
| Acinar | 46 (41.1) |
| Papillary | 26 (23.2) |
| Micropapillary | 3 (2.7) |
| Solid | 9 (8) |
| Lymph node metastasis | |
| Negative | 104 (92.9) |
| Positive | 8 (7.1) |
CEA, carcinoembryonic antigen; VATS, video-assisted thoracic surgery.
Diagnostic Accuracy of FS-A and FS-H&E
Examples of VPI diagnosis by elastic staining under light microscope Image 1A and FS-A under a fluorescence microscope Image 1B are shown. For the FS-A diagnosis, there were nine cases of discrepancy between FS-A diagnosis and postoperative elastic staining examination: five PL1 were misdiagnosed as PL0, and four PL2 were underestimated as PL1 Table 2. The total concordance rate between FS-A and elastic staining was 92%. When cases with PL1 and PL2 were classified together as VPI, the concordance rate was 95.5%. The accuracy, sensitivity, and specificity of FS-A for VPI diagnosis was 95.5%, 86.8%, and 100%, respectively Table 3.
Comparison of Frozen Section and Permanent Section in Diagnosing Visceral Pleural Invasion (n = 112)
| FS Diagnosis . | PE-ES Diagnosis . | . | . |
|---|---|---|---|
| . | PL0 (n = 74) . | PL1 (n = 31) . | PL2 (n = 7) . |
| FS-A, No. (%) | |||
| PL0 | 74 (100) | 5 (16.1) | 0 (0) |
| PL1 | 0 (0) | 26 (83.9) | 4 (57.1) |
| PL2 | 0 (0) | 0 (0) | 3 (42.9) |
| FS-H&E, No. (%) | |||
| PL0 | 72 (97.3) | 19 (61.3) | 1 (14.3) |
| PL1 | 2 (2.7) | 12 (38.7) | 6 (85.7) |
| PL2 | 0 (0) | 0 (0) | 0 (0) |
| FS Diagnosis . | PE-ES Diagnosis . | . | . |
|---|---|---|---|
| . | PL0 (n = 74) . | PL1 (n = 31) . | PL2 (n = 7) . |
| FS-A, No. (%) | |||
| PL0 | 74 (100) | 5 (16.1) | 0 (0) |
| PL1 | 0 (0) | 26 (83.9) | 4 (57.1) |
| PL2 | 0 (0) | 0 (0) | 3 (42.9) |
| FS-H&E, No. (%) | |||
| PL0 | 72 (97.3) | 19 (61.3) | 1 (14.3) |
| PL1 | 2 (2.7) | 12 (38.7) | 6 (85.7) |
| PL2 | 0 (0) | 0 (0) | 0 (0) |
FS-A, frozen section autofluorescence; FS-H&E, frozen section H&E staining; PE-ES: paraffin-embedded tissue section elastic staining.
Comparison of Frozen Section and Permanent Section in Diagnosing Visceral Pleural Invasion (n = 112)
| FS Diagnosis . | PE-ES Diagnosis . | . | . |
|---|---|---|---|
| . | PL0 (n = 74) . | PL1 (n = 31) . | PL2 (n = 7) . |
| FS-A, No. (%) | |||
| PL0 | 74 (100) | 5 (16.1) | 0 (0) |
| PL1 | 0 (0) | 26 (83.9) | 4 (57.1) |
| PL2 | 0 (0) | 0 (0) | 3 (42.9) |
| FS-H&E, No. (%) | |||
| PL0 | 72 (97.3) | 19 (61.3) | 1 (14.3) |
| PL1 | 2 (2.7) | 12 (38.7) | 6 (85.7) |
| PL2 | 0 (0) | 0 (0) | 0 (0) |
| FS Diagnosis . | PE-ES Diagnosis . | . | . |
|---|---|---|---|
| . | PL0 (n = 74) . | PL1 (n = 31) . | PL2 (n = 7) . |
| FS-A, No. (%) | |||
| PL0 | 74 (100) | 5 (16.1) | 0 (0) |
| PL1 | 0 (0) | 26 (83.9) | 4 (57.1) |
| PL2 | 0 (0) | 0 (0) | 3 (42.9) |
| FS-H&E, No. (%) | |||
| PL0 | 72 (97.3) | 19 (61.3) | 1 (14.3) |
| PL1 | 2 (2.7) | 12 (38.7) | 6 (85.7) |
| PL2 | 0 (0) | 0 (0) | 0 (0) |
FS-A, frozen section autofluorescence; FS-H&E, frozen section H&E staining; PE-ES: paraffin-embedded tissue section elastic staining.
| Method . | Accuracy, % . | Sensitivity, % . | Specificity, % . | PPV, % . | NPV, % . |
|---|---|---|---|---|---|
| FS-A | 95.5 | 86.8 | 100 | 100 | 93.7 |
| FS-H&E | 80.4 | 47.4 | 97.3 | 90 | 78.3 |
| Method . | Accuracy, % . | Sensitivity, % . | Specificity, % . | PPV, % . | NPV, % . |
|---|---|---|---|---|---|
| FS-A | 95.5 | 86.8 | 100 | 100 | 93.7 |
| FS-H&E | 80.4 | 47.4 | 97.3 | 90 | 78.3 |
FS-A, frozen section autofluorescence; FS-H&E, frozen section H&E staining; NPV, negative predictive value; PPV, positive predictive value.
| Method . | Accuracy, % . | Sensitivity, % . | Specificity, % . | PPV, % . | NPV, % . |
|---|---|---|---|---|---|
| FS-A | 95.5 | 86.8 | 100 | 100 | 93.7 |
| FS-H&E | 80.4 | 47.4 | 97.3 | 90 | 78.3 |
| Method . | Accuracy, % . | Sensitivity, % . | Specificity, % . | PPV, % . | NPV, % . |
|---|---|---|---|---|---|
| FS-A | 95.5 | 86.8 | 100 | 100 | 93.7 |
| FS-H&E | 80.4 | 47.4 | 97.3 | 90 | 78.3 |
FS-A, frozen section autofluorescence; FS-H&E, frozen section H&E staining; NPV, negative predictive value; PPV, positive predictive value.
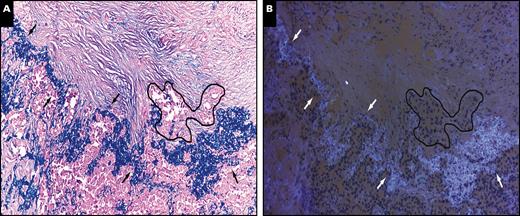
Example of visceral pleural invasion diagnosis by elastic staining under a light microscope (A) and frozen section autofluorescence under a fluorescence microscope (B) from two serial tissue sections. Arrows indicate the elastic layer of the visceral pleural and the black line indicates the cluster of tumor cells invading beyond the elastic layer. (A and B, ×100).
However, there were 28 cases of discrepancy between FS-H&E diagnosis and elastic staining examination. Most discrepant cases were PL1 misdiagnosed as PL0 (67.9%, 19/28). The remaining discrepancies were as follows: two PL0 misdiagnosed as PL1, one PL2 misdiagnosed as PL0, and six PL2 underestimated as PL1 (Table 2). The total concordance rate between FS-H&E and elastic staining was only 75%. When cases with PL1 and PL2 were classified together as VPI, the concordance rate was 80.4%. The accuracy, sensitivity, and specificity of FS-H&E for VPI diagnosis was 80.4%, 47.4%, and 97.3%, respectively (Table 3).
Diagnostic Reproducibility Analysis
For intraobserver reproducibility, there was substantial agreement on VPI diagnosis (κ = 0.82): the same diagnosis was made in 92% (46/50) of the additional examinations of slides 3 months later. With regard to interobserver reproducibility, there was also substantial agreement on VPI diagnosis (100/112), with a κ value of 0.77 between the two pathologists (H.X. and S.Z.).
Reasons for Misdiagnosis by Frozen Section Autofluorescence
We found that sampling error accounted for 33.3% (3/9) of the errors. The other six misdiagnosed cases were due to interpretation error: two cases were with only very rare tumor cells invading beyond the elastic layer, and it was difficult to distinguish between PL0 and PL1, even after the elastic staining; four cases had obvious desmoplasia in the visceral pleural, giving excess extracellular collagen which produce an irregular blue fluorescence as background interference. It is worth noting that fluorescence intensity of the elastic layer was stronger and present as a wave shape; however, the nonspecific fluorescence generated by desmoplasia was present as an irregular shape (Supplemental Figure 2).
Prognostic Evaluation Cohort
Baseline Information
The clinicopathologic characteristics of the 339 patients used for prognostic evaluation are shown in Supplemental Table 1. VPI positivity was more frequently identified in patients with larger tumor size (10-20 mm, 86.8% vs 70.3%; P = .013), higher grade of histologic pattern (solid pattern, 20.8% vs 7.7%; P = .009), and lymph node metastasis (13.2% vs 5.6%; P = .043).
Prognostic Impact of VPI in Patients Treated With Sublobar Resection
The 5-year RFS rate was significantly better in patients with VPI negativity than patients with VPI positivity (84.3% vs 71.7%; P = .014) Figure 2A. Moreover, the 5-year RFS rate of patients who had undergone lobectomy was significantly better than for patients who had undergone sublobar resection (81.6% vs 77.1%; P = .018) Figure 2B. When patients were divided into VPI-negative and VPI-positive subgroups, there was no differences in RFS between lobectomy and sublobar resections in the VPI-negative subgroup (5-year RFS rate, 84.8% vs 81.8%; P = .215) Figure 2C. However, in the VPI-positive subgroup, lobectomy produced a significantly better prognosis compared to sublobar resection (5-year RFS rate, 80.0% vs 50%; P = .009) Figure 2D.
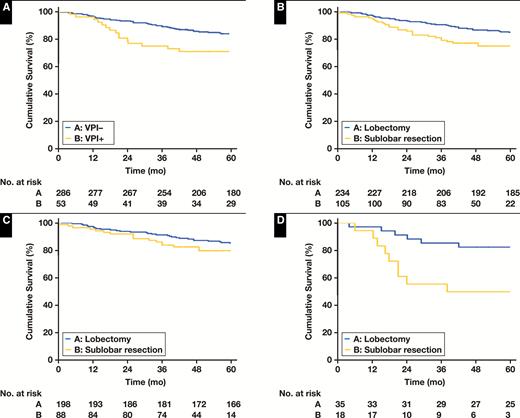
Recurrence-free survival (RFS) in patients with lung adenocarcinoma 2 cm or less stratified by visceral pleural invasion (VPI) and surgical procedure. A, RFS by VPI in all patients; P = .014. B, RFS by surgical procedure in all patients; P = .018. C, RFS by surgical procedure for patients with VPI negativity (n = 286); P = .215. D, RFS by surgical procedure for patients with VPI positivity (n = 53); P = .009.
Multivariate Cox analysis showed that sublobar resection (hazard ratio [HR], 3.30; 95% confidence interval [CI], 1.18-9.25; P = .023) was the only independent risk factor for RFS in the VPI-positive subgroup. In the VPI-negative subgroup, larger tumor size (10-20 mm vs 0-10 mm, HR, 2.21; 95% CI, 1.08-4.52; P = .023) and lymph node metastasis (HR, 3.49; 95% CI, 1.77-6.91; P < .001) were the independent risk factors for RFS; however, sublobar resection was not an independent risk factor Table 4.
Cox Proportional-Hazards Regression Model for Recurrence-Free Survival in Patients With Lung Adenocarcinoma 2 cm or Less Who Underwent Surgical Resectiona
| Variables . | Recurrence-Free Survival . | . | . |
|---|---|---|---|
| . | Univariate Analysis . | Multivariate Analysis . | . |
| . | P Value . | HR (95% CI) . | P Value . |
| VPI-negative subgroup (n = 286) | |||
| Tumor size, 10-20 mm vs 0-10 mm | .034 | 2.21 (1.08-4.52) | .031 |
| CEA, >5 ng/mL vs ≤5 ng/mL | .209 | ||
| Tumor location, lower vs upper and middle | .629 | ||
| Surgical procedure, sublobar resection vs lobectomy | .218 | ||
| Lepidic predominant, yes vs no | .010 | 0.60 (0.31-1.16) | .127 |
| Acinar predominant, yes vs no | .988 | ||
| Papillary predominant, yes vs no | .205 | ||
| Micropapillary predominant, yes vs no | .697 | ||
| Solid predominant, yes vs no | .017 | 1.19 (0.56-2.52) | .654 |
| LN metastasis, present vs absent | <.001 | 3.49 (1.77-6.91) | <.001 |
| VPI-positive subgroup (n = 53) | |||
| Tumor size, 10-20 mm vs 0-10 mm | .742 | ||
| CEA, >5 ng/mL vs ≤5 ng/mL | .875 | ||
| Tumor location, lower vs upper and middle | .169 | ||
| Surgical procedure, sublobar resection vs lobectomy | .015 | 3.30 (1.18-9.25) | .023 |
| Lepidic predominant, yes vs no | .854 | ||
| Acinar predominant, yes vs no | .133 | ||
| Papillary predominant, yes vs no | .863 | ||
| Solid predominant, yes vs no | .055 | 2.38 (0.85-6.63) | .098 |
| LN metastasis, present vs absent | .147 |
| Variables . | Recurrence-Free Survival . | . | . |
|---|---|---|---|
| . | Univariate Analysis . | Multivariate Analysis . | . |
| . | P Value . | HR (95% CI) . | P Value . |
| VPI-negative subgroup (n = 286) | |||
| Tumor size, 10-20 mm vs 0-10 mm | .034 | 2.21 (1.08-4.52) | .031 |
| CEA, >5 ng/mL vs ≤5 ng/mL | .209 | ||
| Tumor location, lower vs upper and middle | .629 | ||
| Surgical procedure, sublobar resection vs lobectomy | .218 | ||
| Lepidic predominant, yes vs no | .010 | 0.60 (0.31-1.16) | .127 |
| Acinar predominant, yes vs no | .988 | ||
| Papillary predominant, yes vs no | .205 | ||
| Micropapillary predominant, yes vs no | .697 | ||
| Solid predominant, yes vs no | .017 | 1.19 (0.56-2.52) | .654 |
| LN metastasis, present vs absent | <.001 | 3.49 (1.77-6.91) | <.001 |
| VPI-positive subgroup (n = 53) | |||
| Tumor size, 10-20 mm vs 0-10 mm | .742 | ||
| CEA, >5 ng/mL vs ≤5 ng/mL | .875 | ||
| Tumor location, lower vs upper and middle | .169 | ||
| Surgical procedure, sublobar resection vs lobectomy | .015 | 3.30 (1.18-9.25) | .023 |
| Lepidic predominant, yes vs no | .854 | ||
| Acinar predominant, yes vs no | .133 | ||
| Papillary predominant, yes vs no | .863 | ||
| Solid predominant, yes vs no | .055 | 2.38 (0.85-6.63) | .098 |
| LN metastasis, present vs absent | .147 |
aMultivariable Cox model was adjusted by age, gender, and smoking status.
CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; LN, lymph node; VPI, visceral pleural invasion.
Cox Proportional-Hazards Regression Model for Recurrence-Free Survival in Patients With Lung Adenocarcinoma 2 cm or Less Who Underwent Surgical Resectiona
| Variables . | Recurrence-Free Survival . | . | . |
|---|---|---|---|
| . | Univariate Analysis . | Multivariate Analysis . | . |
| . | P Value . | HR (95% CI) . | P Value . |
| VPI-negative subgroup (n = 286) | |||
| Tumor size, 10-20 mm vs 0-10 mm | .034 | 2.21 (1.08-4.52) | .031 |
| CEA, >5 ng/mL vs ≤5 ng/mL | .209 | ||
| Tumor location, lower vs upper and middle | .629 | ||
| Surgical procedure, sublobar resection vs lobectomy | .218 | ||
| Lepidic predominant, yes vs no | .010 | 0.60 (0.31-1.16) | .127 |
| Acinar predominant, yes vs no | .988 | ||
| Papillary predominant, yes vs no | .205 | ||
| Micropapillary predominant, yes vs no | .697 | ||
| Solid predominant, yes vs no | .017 | 1.19 (0.56-2.52) | .654 |
| LN metastasis, present vs absent | <.001 | 3.49 (1.77-6.91) | <.001 |
| VPI-positive subgroup (n = 53) | |||
| Tumor size, 10-20 mm vs 0-10 mm | .742 | ||
| CEA, >5 ng/mL vs ≤5 ng/mL | .875 | ||
| Tumor location, lower vs upper and middle | .169 | ||
| Surgical procedure, sublobar resection vs lobectomy | .015 | 3.30 (1.18-9.25) | .023 |
| Lepidic predominant, yes vs no | .854 | ||
| Acinar predominant, yes vs no | .133 | ||
| Papillary predominant, yes vs no | .863 | ||
| Solid predominant, yes vs no | .055 | 2.38 (0.85-6.63) | .098 |
| LN metastasis, present vs absent | .147 |
| Variables . | Recurrence-Free Survival . | . | . |
|---|---|---|---|
| . | Univariate Analysis . | Multivariate Analysis . | . |
| . | P Value . | HR (95% CI) . | P Value . |
| VPI-negative subgroup (n = 286) | |||
| Tumor size, 10-20 mm vs 0-10 mm | .034 | 2.21 (1.08-4.52) | .031 |
| CEA, >5 ng/mL vs ≤5 ng/mL | .209 | ||
| Tumor location, lower vs upper and middle | .629 | ||
| Surgical procedure, sublobar resection vs lobectomy | .218 | ||
| Lepidic predominant, yes vs no | .010 | 0.60 (0.31-1.16) | .127 |
| Acinar predominant, yes vs no | .988 | ||
| Papillary predominant, yes vs no | .205 | ||
| Micropapillary predominant, yes vs no | .697 | ||
| Solid predominant, yes vs no | .017 | 1.19 (0.56-2.52) | .654 |
| LN metastasis, present vs absent | <.001 | 3.49 (1.77-6.91) | <.001 |
| VPI-positive subgroup (n = 53) | |||
| Tumor size, 10-20 mm vs 0-10 mm | .742 | ||
| CEA, >5 ng/mL vs ≤5 ng/mL | .875 | ||
| Tumor location, lower vs upper and middle | .169 | ||
| Surgical procedure, sublobar resection vs lobectomy | .015 | 3.30 (1.18-9.25) | .023 |
| Lepidic predominant, yes vs no | .854 | ||
| Acinar predominant, yes vs no | .133 | ||
| Papillary predominant, yes vs no | .863 | ||
| Solid predominant, yes vs no | .055 | 2.38 (0.85-6.63) | .098 |
| LN metastasis, present vs absent | .147 |
aMultivariable Cox model was adjusted by age, gender, and smoking status.
CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; LN, lymph node; VPI, visceral pleural invasion.
Discussion
To the best of our knowledge, this is the first study investigating the use of autofluorescence for diagnosis of VPI in frozen sections under a fluorescence microscope. Accurate intraoperative diagnosis for VPI is helpful for surgical decision making during operation.
The surgical procedure for small-sized lung adenocarcinoma is a controversial topic among thoracic surgeons. Even so, up to 20% to 30% of small-size invasive lung adenocarcinomas are treated by sublobar resection based on large studies from SEER and NCDB, as well as European and east Asian institutes.3-6 Additionally, our previous study demonstrated that VPI was visible even in lung adenocarcinomas manifesting as ground-glass opacities (GGNs), which are often managed by a sublobar resection.20 Therefore, for tumors with VPI, even if the size is 2 cm or less or manifesting as GGNs, the surgical procedure should not be a sublobar resection with insufficient lymph node dissection.
Currently, the accuracy of predicting VPI via intraoperative macroscopic and preoperative imaging tests such as CT and magnetic resonance imaging is not satisfactory as accuracy ranges from 71% to 83.3%.21-23 Up to now, there have only been two studies concerning intraoperative VPI diagnoses using either photodynamic diagnosis with an autofluorescence observation system or the autofluorescence mode of a thoracoscope.13,24 Our study offers another alternative for VPI diagnoses in frozen sections, which is not only time saving but also effort saving.
Although elastic staining is accurate to evaluate VPI, the process takes a relatively long time and needs formalin-fixed paraffin-embedded tissue. Hence, it is almost impossible to achieve in the operation. Our technology has practical implications for the management of patients with small-size lung adenocarcinomas, especially those tumors with diameter 2 cm or less or manifesting as GGNs on CT. Autofluorescence is most applicable and reasonable to use in those two populations, which are potentially candidates for intentional limited resection.1,25 Accurate intraoperative diagnosis for VPI in lung adenocarcinoma is helpful for surgical decision making. When intraoperative frozen sections show positive findings for VPI, in a planned sublobar resection for a tumor with diameter 2 cm or less or manifesting as GGNs, an additional lobectomy with lymphadenectomy should be performed. According to National Comprehensive Cancer Network Lung Cancer Resection Quality Criteria, the examination of at least three mediastinal lymph node stations is necessary. Hence, standard hilar and mediastinal lymph node dissection should be required in patients with VPI positivity, because VPI is reported to be a risk factor for skip N2 metastases.14,26
We must acknowledge some limitations of our study. First, the survival data of the patients who underwent surgery in 2017 were not mature enough to perform a survival analysis; thus, patients who underwent surgery from 2009 to 2010 were identified for prognostic evaluation. Second, we included only patients from a single institution; thus, potential bias could be inevitable and external validation is needed to further confirm our method’s accuracy. Third, cases with obvious desmoplasia in the visceral pleural produce an irregular blue fluorescence that caused interference, and single cells invading pleura were also difficult to identify. However, the fluorescence intensity of the elastic layer was stronger and present as a wave shape, which is helpful for distinguishing from nonspecific background fluorescence. Hence, skilled use of this diagnostic technique requires a learning process to identify the wavy fluorescence of elastic fibers.
In conclusion, using autofluorescence from frozen sections appears to be an accurate method for diagnosing VPI, which is helpful for surgical decision making.
Acknowledgment
This work was supported by the Shanghai Hospital Development Center (grant numbers SHDC12015116 and SHDC16CR4026A), Fundamental Research Funds for the Central Universities (grant number 22120180607), National Natural Science Foundation of China (grant number 81802256), Science and Technology Commission of Shanghai Municipality (grant numbers 15411968400 and 14411962600), Shanghai Municipal Education Commission and Shanghai Education Development Foundation (grant number 18CG19), and Shanghai Pujiang Program (grant number 15PJD034).
References
Author notes
First authors.




